“Underwater Acoustic Communication Market”
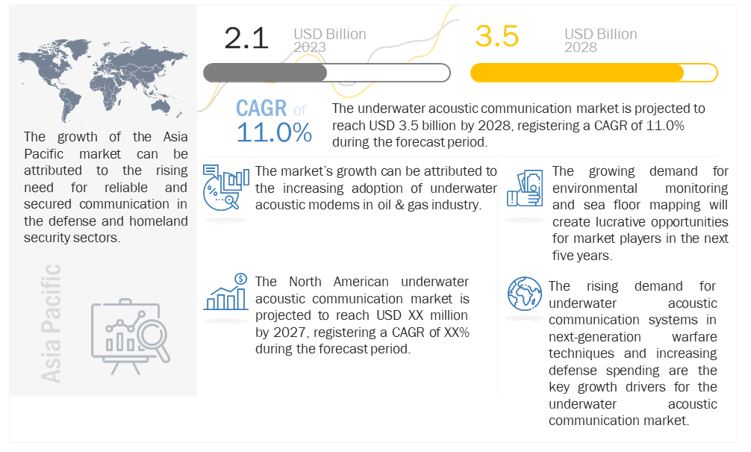
The Underwater Acoustic Communication Market Size is expected to reach USD 3.5 billion by 2028 from USD 2.1 billion in 2023 growing at a CAGR of 11.0% during forecast year.
CHICAGO, Dec 04, 2023 – The global Underwater Acoustic Communication Market is experiencing substantial growth, with a current valuation of USD 2.1 billion in 2023 and an anticipated increase to USD 3.5 billion by 2028, reflecting a robust Compound Annual Growth Rate (CAGR) of 11.0% during the forecast period. These insights are detailed in a comprehensive report by MarketsandMarkets, a leading market research and consulting firm.
Applications like as offshore oil and gas development, environmental monitoring, defence and security, and marine research heavily rely on underwater acoustic communication technology. The growing need for dependable underwater communication solutions, developments in sonar technology, and the growing use of autonomous underwater vehicles (AUVs) for underwater exploration are the main drivers of the market’s anticipated expansion. The study analyses major competitors in the global underwater acoustic communication market and examines important market segments, growth factors, obstacles, and prospects.
• Informational PDF Brochure:- https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=259232207
Key Segments:
Communication Range Segment: The Underwater Acoustic Communication market includes various communication ranges, such as short-range, medium-range, and long-range communication, catering to different underwater applications.
End-User Segment: The market serves various end users, including oil and gas companies, defense and security agencies, research institutions, and environmental monitoring organizations, each with specific requirements for underwater communication solutions.
Application Segment: Underwater acoustic communication finds applications in diverse sectors, including offshore oil and gas exploration, environmental monitoring, marine research, defense, and surveillance, reflecting the broad spectrum of underwater communication needs.
Browse 153 market data Tables and 52 Figures spread through 199 Pages and in-depth TOC on “Underwater Acoustic Communication Market by Interface Platform, Application, Communication Depth, End User and Region – Global Forecast to 2028”
View detailed Table of Content here – https://www.marketsandmarkets.com/Market-Reports/underwater-acoustic-communication-market-259232207.html
Growth Drivers:
Increasing Offshore Activities: The growing demand for offshore oil and gas exploration and production activities drives the need for reliable and efficient underwater communication solutions.
Advancements in Sonar Technologies: Ongoing advancements in sonar technologies, including underwater acoustic modems and transducers, contribute to the improvement of underwater communication capabilities.
Rising Adoption of AUVs: The increasing adoption of autonomous underwater vehicles (AUVs) for underwater exploration and surveillance creates a demand for effective underwater communication systems.
Challenges:
Limited Data Transfer Rates: The limited data transfer rates in underwater environments pose challenges for real-time communication and data transmission.
Environmental Interference: Factors such as water temperature, salinity, and marine life can impact the performance of underwater acoustic communication systems, posing challenges for reliability.
Opportunities:
Underwater IoT Applications: The integration of underwater acoustic communication with the Internet of Things (IoT) opens opportunities for monitoring and collecting data from underwater sensors.
Marine Research and Exploration: The expansion of marine research and exploration activities, including deep-sea exploration, presents opportunities for the deployment of advanced underwater communication solutions.
Key Players:
The report identifies key players in the global Underwater Acoustic Communication market, including:
Teledyne Technologies Incorporated: A leading provider of underwater acoustic communication solutions, including modems and hydrophones for marine applications.
Kongsberg Gruppen: A multinational company specializing in underwater technology, offering acoustic modems, transceivers, and communication systems for marine and defense applications.
Thales Group: A global technology company providing underwater communication solutions for defense, marine, and offshore applications.
L3Harris Technologies, Inc.: A multinational aerospace and defense company offering communication and sonar solutions for underwater applications.
Sonardyne International Ltd.: A UK-based company specializing in underwater technology, providing acoustic communication and positioning solutions for marine and subsea industries.
The growing need for dependable communication solutions in underwater environments is expected to propel significant growth in the global underwater acoustic communication market. Underwater acoustic communication systems are anticipated to be essential to many different applications, including environmental monitoring and offshore exploration, as technology develops.
Media Contact
Company Name: MarketsandMarkets™ Research Private Ltd.
Contact Person: Mr. Aashish Mehra
Email: Send Email
Phone: 18886006441
Address:630 Dundee Road Suite 430
City: Northbrook
State: IL 60062
Country: United States
Website: https://www.marketsandmarkets.com/Market-Reports/underwater-acoustic-communication-market-259232207.html
Press Release Distributed by ABNewswire.com
To view the original version on ABNewswire visit: Underwater Acoustic Communication Market Making Waves, Poised to Reach USD 3.5 Billion by 2028, at a CAGR of 11.0%