InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the“Global Medical Device Analytical Testing Outsourcing Market Size, Share & Trends Analysis Report By Service (Extractable and Leachable, Material Characterization, Physical Testing, Bioburden Testing, Sterility Testing), Device Type (Reprocessed Devices), End Use (Hospitals, Others)- Market Outlook And Industry Analysis 2031″
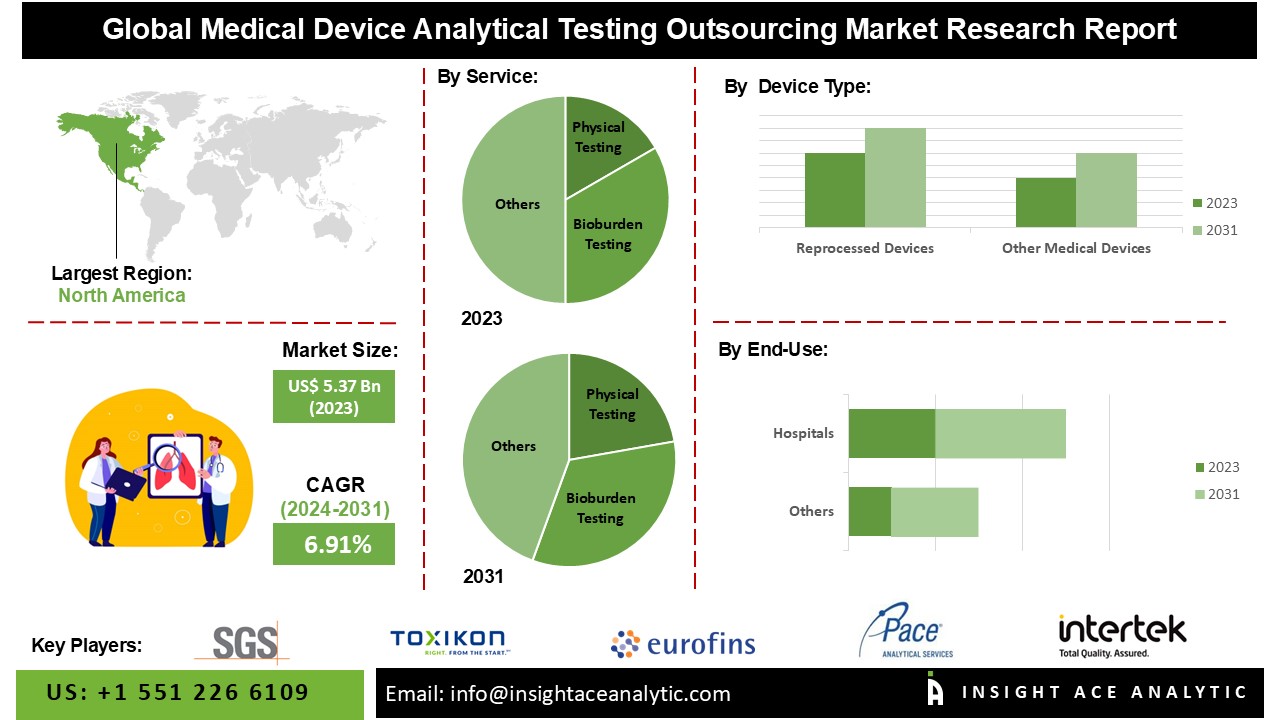
The Global Medical Device Analytical Testing Outsourcing Market is valued at US$ 5.37 Bn in 2023, and it is expected to reach US$ 8.98 Bn by 2031, with a CAGR of 6.91% during the forecast period of 2024-2031.
Get Free Access to Demo Report, Excel Pivot and ToC : https://www.insightaceanalytic.com/request-sample/2677
The Medical Device Analytical Testing Outsourcing Market is set for significant growth, fueled by the increasing complexity of medical devices and the stringent regulatory environment governing their approval and use. Analytical testing in this sector involves various critical evaluations, including extractable and leachable testing, material characterization, physical testing, bioburden testing, and sterility testing, all aimed at ensuring the safety, efficacy, and quality of medical devices before they reach the market. These assessments are crucial across multiple fields, such as drug delivery systems, orthopedics, cardiovascular devices, and diagnostic imaging, helping to verify that devices meet safety standards and function as intended in clinical settings.
The importance of these testing services lies not only in regulatory compliance but also in mitigating risks associated with product failures, which can have severe implications for patient safety and brand reputation. Outsourcing analytical testing has become a strategic choice for many medical device manufacturers, enabling them to access specialized expertise and advanced technologies without the substantial investment required for in-house testing facilities. This approach allows companies to focus on their core competencies while ensuring their products meet rigorous safety and quality standards.
List of Prominent Players in the Medical Device Analytical Testing Outsourcing Market:
- SGS
- Toxikon, Inc.
- Eurofins Scientific
- Pace Analytical Services LLC
- Intertek Group plc
- Wuxi AppTec
- North American Science Associates, Inc.
- Envigo
- Charles River Laboratories International, Inc.
- Medical Device Testing Services
Expert Knowledge, Just a Click Away: https://calendly.com/insightaceanalytic/30min?month=2024-02
Market Dynamics:
Drivers-
The growth of the Medical Device Analytical Testing Outsourcing Market is driven by increasing demand for medical devices, stringent regulatory requirements, cost efficiency, and technological advancements. The rising prevalence of chronic diseases like diabetes and cardiovascular conditions heightens the need for reliable and safe medical devices, fueling demand for comprehensive analytical testing to ensure compliance and safety. Regulatory bodies like the FDA and EMA impose strict guidelines, prompting manufacturers to outsource testing to specialized laboratories for efficiency and compliance. Outsourcing also offers cost savings and faster time-to-market by eliminating the need for expensive in-house testing facilities. Additionally, advancements in analytical technologies, such as mass spectrometry and chromatography, enhance testing accuracy and efficiency, further driving the market’s growth.
Challenges:
Price sensitivity in developing countries, data security concerns, and regulatory compliance challenges are key impediments to the growth of the Medical Device Analytical Testing Outsourcing Market. In developing nations, budget constraints make adoption of outsourced testing services difficult. Additionally, manufacturers are cautious about data security risks when sharing sensitive information with third-party labs, adding complexity and costs. Ensuring that outsourced laboratories meet stringent regulatory standards across different jurisdictions further complicates compliance, making it resource-intensive for manufacturers.
Regional Trends:
North America leads the Medical Device Analytical Testing Outsourcing Market due to its stringent regulatory environment, advanced healthcare infrastructure, presence of key industry players, focus on innovation, and cost efficiency. The region’s rigorous FDA regulations drive the need for comprehensive testing, compelling manufacturers to outsource to specialized labs that excel in compliance. North America’s well-developed infrastructure supports the rapid adoption of innovative testing technologies, while key players like SGS SA and Eurofins Scientific invest in R&D to provide high-quality services. Additionally, the region’s emphasis on medical innovation fuels demand for advanced testing, and outsourcing offers significant cost savings and faster time-to-market.
Empower Your Decision-Making with 180 Pages Full Report @ https://www.insightaceanalytic.com/enquiry-before-buying/2677
Recent Developments:
- In July 2024, SGS North America (SGS) has expanded its biologics testing services at the SGS Lincolnshire Center of Excellence, enhancing its capacity and capabilities to better serve the American biopharmaceutical market with advanced instrumentation and scientific expertise.
- In March 2024, Pace Analytical Services LLC acquired the Lebanon, New Jersey laboratory facility from Curia.
Segmentation of Medical Device Analytical Testing Outsourcing Market-
By Service:
- Extractable and Leachable
- Material Characterization
- Physical Testing
- Bioburden Testing
- Sterility Testing
- Other Tests
By Device Type:
- Reprocessed Devices
- Other Medical Devices
By End Use:
- Hospitals
- Others
By Region-
North America-
- The US
- Canada
- Mexico
Europe-
- Germany
- The UK
- France
- Italy
- Spain
- Rest of Europe
Asia-Pacific-
- China
- Japan
- India
- South Korea
- South East Asia
- Rest of Asia Pacific
Latin America-
- Brazil
- Argentina
- Rest of Latin America
Middle East & Africa-
- GCC Countries
- South Africa
- Rest of Middle East and Africa
Unlock Your GTM Strategy: https://www.insightaceanalytic.com/customisation/2677
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
Media Contact
Company Name: InsightAce Analytic Pvt. Ltd
Contact Person: Diana D’Souza
Email: Send Email
Country: United States
Website: https://www.insightaceanalytic.com/
Press Release Distributed by ABNewswire.com
To view the original version on ABNewswire visit: Medical Device Analytical Testing Outsourcing: Market Trends, Challenges, and Opportunities