DelveInsight’s “Chronic Hepatitis B Market Insights, Epidemiology, and Market Forecast 2034” report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the Chronic Hepatitis B market size, share, trends, and growth opportunities in the seven major markets (7MM) (i.e., the United States, EU4 (Germany, Spain, Italy, France), the United Kingdom and Japan).
The Chronic Hepatitis B market report covers emerging drugs, current treatment practices, market share of individual therapies, and current & forecasted market size from 2020 to 2034. It also evaluates the current treatment practice/algorithm, key drivers & barriers impacting the market growth, and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
Chronic Hepatitis B Overview
Chronic Hepatitis B (CHB) is a long-term infection caused by the Hepatitis B virus (HBV) that can lead to serious liver damage and potentially life-threatening complications. It is a major global health issue, affecting millions of people worldwide. CHB is characterized by the persistence of the virus in the body for more than six months, leading to ongoing inflammation and potential scarring of the liver.
Key Features of Chronic Hepatitis B
1. Persistent Viral Infection: CHB is defined by the presence of HBV in the body for more than six months, often leading to a chronic carrier state.
2. Variable Clinical Course: The disease can range from asymptomatic to active liver inflammation, with varying degrees of liver damage.
3. Risk of Complications: Long-term infection can lead to cirrhosis, liver failure, and an increased risk of liver cancer.
Causes of Chronic Hepatitis B
CHB is primarily caused by the Hepatitis B virus, a DNA virus that is transmitted through contact with infected blood or body fluids. Key routes of transmission include:
– Mother-to-Child: Vertical transmission from an infected mother to her child during birth.
– Unsafe Injections and Medical Procedures: Use of contaminated needles or medical equipment.
Diagnosis and Treatment
Diagnosing CHB involves a combination of blood tests and liver function tests:
– HBV Surface Antigen (HBsAg): A positive result indicates an ongoing infection.
– HBV DNA Levels: Quantitative tests measure the amount of viral DNA in the blood, indicating the level of viral replication.
– Liver Function Tests: Elevated liver enzymes can indicate liver inflammation or damage.
– Liver Biopsy: In some cases, a liver biopsy may be performed to assess the extent of liver damage.
Treatment for CHB aims to suppress viral replication, reduce the risk of complications, and improve long-term outcomes. Options include:
– Antiviral Medications: Drugs such as nucleos(t)ide analogues (e.g., entecavir, tenofovir) are used to inhibit viral replication.
– Interferon Therapy: Pegylated interferon can be used in certain cases, although it is less commonly used due to its side effects and limited efficacy.
– Monitoring and Management: Regular monitoring of liver function and viral load is essential to manage the disease effectively.
Prognosis
The prognosis for individuals with CHB varies widely depending on factors such as the level of viral replication, the degree of liver damage, and the presence of other risk factors (e.g., alcohol use, co-infection with Hepatitis C or HIV). Early diagnosis and treatment can significantly reduce the risk of complications and improve long-term outcomes.
Chronic Hepatitis B is a complex and potentially severe liver disease that requires careful management. Advances in treatment and ongoing research continue to improve outcomes for affected individuals. It is crucial for those at risk to undergo screening and for those diagnosed to receive appropriate care and monitoring.
Key highlights from the Chronic Hepatitis B Market Report:
-
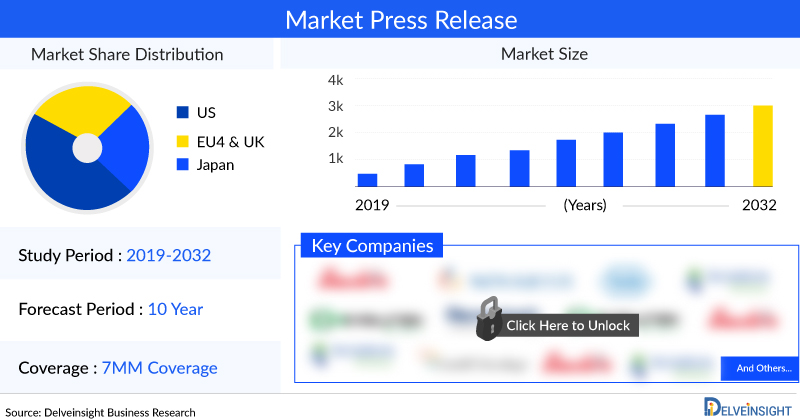
In 2021, the total chronic hepatitis B market size was USD 2,605 million, which is expected to rise during the study period (2020–2034).
-
The total market size of chronic hepatitis B in the United States was USD 1,741 million in 2021.
-
In EU5, the total market size of chronic hepatitis B was USD 483 million in 2021.
-
In Japan, the total market size of chronic hepatitis B was USD 380 million in 2021.
-
In the year 2021, the total diagnosed prevalent cases of chronic hepatitis B were 2,165,800+ cases in the 7MM, which is expected to grow during the study period, i.e., 2020–2034.
-
According to the World Health Organization (WHO) estimates, in 2015, 257 million people were living with chronic hepatitis B infection. Also, as of 2016, 27 million people (10.5% of all people estimated to be living with hepatitis B) were aware of their infection, while 4.5 million (16.7%) of the people diagnosed were on treatment.
The market outlook section of the report helps to build a detailed comprehension of the historical, current and forecasted Chronic Hepatitis B market size by analyzing the impact of current and emerging pipeline therapies. It also thoroughly assesses the market drivers & barriers, unmet needs, and emerging technologies set to impact the market dynamics.
The report gives complete detail of the Chronic Hepatitis B market trend for each marketed drug and mid & late-stage pipeline therapies by evaluating their impact based on the annual cost of therapy, their Mechanism of Action (MOA), Route of Administration (ROA), molecule types, competition with other therapies, brand value, and their impact on the market.
Chronic Hepatitis B Epidemiology Assessment
The epidemiology section provides insights into the historical, current, and forecasted epidemiology trends in the seven major countries (7MM) from 2020 to 2034. It helps to recognize the causes of current and forecasted epidemiology trends by exploring numerous studies and research. The epidemiology section also provides a detailed analysis of diagnosed and prevalent patient pool, future trends, and views of key opinion leaders.
The Report Covers the Chronic Hepatitis B Epidemiology, Segmented as –
-
Prevalence population of CHB in the 7MM (2020–2034)
-
Diagnosed prevalent Cases of CHB in the 7MM (2020–2034)
-
Age-specific Cases of CHB in the 7MM (2020–2034)
-
Gender-specific Cases of CHB in the 7MM (2020–2034)
-
Type-specific Cases of CHB in the 7MM (2020–2034)
-
Total Treated cases of CHB in the 7MM (2020–2034)
Chronic Hepatitis B Drugs Uptake and Pipeline Development Activities
The drug uptake section focuses on the uptake rate of potential drugs recently launched in the Chronic Hepatitis B market or expected to be launched during the study period. The analysis covers the Chronic Hepatitis B market uptake by drugs, patient uptake by therapies, and sales of each drug. Moreover, the therapeutics assessment section helps understand the drugs with the most rapid uptake and the reasons behind the maximal use of the drugs. Additionally, it compares the drugs based on market share.
The report also covers the Chronic Hepatitis B pipeline development activities. It provides valuable insights about different therapeutic candidates in various stages and the key companies involved in developing targeted therapeutics. It also analyses recent developments such as collaborations, acquisitions, mergers, licensing patent details, and other information for emerging therapies.
Learn How the Chronic Hepatitis B Market Will Evolve and Grow by 2034
Chronic Hepatitis B Therapeutics Analysis
The goals of treatment for chronic hepatitis B virus infection are to reduce liver inflammation and prevent complications by suppressing viral replication. Treatment can slow the progression of cirrhosis, reduce the incidence of liver cancer and improve long-term survival.
Several major pharma and biotech companies are actively working in the market to improve the Chronic Hepatitis B Virus Infection treatment scenario. Currently, Ligand Pharmaceuticals is leading the therapeutics market with its Chronic Hepatitis B Virus Infection drug candidates in the most advanced stage of clinical development
The Leading Companies in the Chronic Hepatitis B Virus Therapeutics Market Include:
Antios Therapeutics, Arbutus Biopharma, Ascentage Pharma, Ascletis Pharmaceuticals, Assembly Biosciences, Brii Biosciences, Chong Kun Dang Pharmaceutical, Dong-A ST Co, Enanta Pharmaceuticals, Enyo Pharma, Gilead Sciences, GlaxoSmithKline, Golden Biotechnology, Guangzhou Lupeng Pharmaceutical, Henlix, Ionis Pharma, Janssen Sciences, Jiangsu HengRui Medicine, Nucorion Pharmaceuticals, PharmaEssentia, Qilu Pharmaceutical, Roche, Romark Laboratories, Shanghai HEP Pharmaceutical, Sunshine Lake Pharma, Suzhou Ribo Life Science, Tasly Tianjin Biopharmaceutical, Vaccitech limited, Vaxine Pty Ltd, Vedanta Biosciences, VenatoRx Pharmaceuticals, Vir Biotechnology, Zhejiang Palo Alto Pharmaceuticals, Zhimeng Biopharm, and others.
Chronic Hepatitis B Virus Infection Emerging and Marketed Drugs Covered in the Report Include:
-
GS-9688 (Selgantolimod): Gilead Sciences
-
GSK3228836 (IONIS-HBVRx): GlaxoSmithKline/Ionis Pharma
-
JNJ-56136379 (JNJ-6379): Janssen Sciences Ireland
-
Pradefovir: Ligand Pharmaceuticals
-
RG6346: Dicerna Pharmaceuticals
-
RG7854 and RG7907 Combination: Roche
-
VIR-2218 and VIR-3434: Vir Biotechnology
-
VTP-300: Vaccitech
And Many More
The Report Covers the In-depth Assessment of the Emerging Drugs & Key Companies. Download the Sample Report to Learn More
Table of Contents
1. Key Insights
2. Executive Summary
3. Chronic Hepatitis B Competitive Intelligence Analysis
4. Chronic Hepatitis B Market Overview at a Glance
5. Chronic Hepatitis B Disease Background and Overview
6. Chronic Hepatitis B Patient Journey
7. Chronic Hepatitis B Epidemiology and Patient Population (In the US, EU5, and Japan)
8. Chronic Hepatitis B Treatment Algorithm, Current Treatment, and Medical Practices
9. Chronic Hepatitis B Unmet Needs
10. Key Endpoints of Chronic Hepatitis B Treatment
11. Chronic Hepatitis B Marketed Products
12. Chronic Hepatitis B Emerging Drugs and Latest Therapeutic Advances
13. Chronic Hepatitis B Seven Major Market Analysis
14. Attribute Analysis
15. Chronic Hepatitis B Market Outlook (In US, EU5, and Japan)
16. Chronic Hepatitis B Access and Reimbursement Overview
17. KOL Views on the Chronic Hepatitis B Market
18. Chronic Hepatitis B Market Drivers
19. Chronic Hepatitis B Market Barriers
20. Appendix
21. DelveInsight Capabilities
22. Disclaimer
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email: Send Email
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/
Press Release Distributed by ABNewswire.com
To view the original version on ABNewswire visit: Chronic Hepatitis B Market to Exhibit Moderate Growth Rate During the Forecast Period | Gilead, Ionis, GSK, Janssen, Dicerna, Roche, Vir Biotech