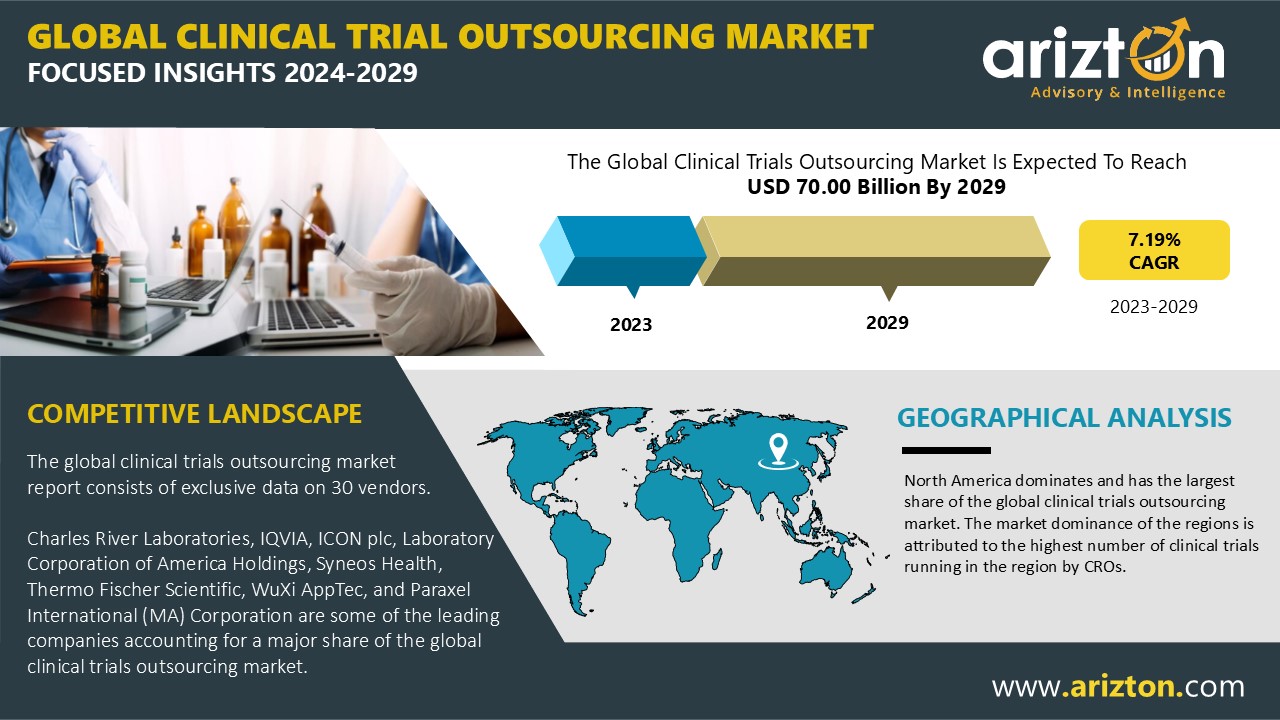
According to Arizton’s latest research report, the global clinical trials outsourcing market is growing at a CAGR of 7.19% during 2023-2029.
Looking for More Information? Click: https://www.arizton.com/market-reports/clinical-trials-outsourcing-market
Report Scope:
Market Size (2029): $70 Billion
Market Size (2023): $46.15 Billion
CAGR (2023-2029): 7.19%
Historic Year: 2020-2022
Base Year: 2023
Forecast Year: 2024-2029
Market Segmentation: Outsourcing Type, Phase, Indications, End-Users and Geography
Geographical Analysis: Europe, North America, APAC, Latin America, and Middle East & Africa
The Growth of Specialized CROs Creating the Global Clinical Trials Outsourcing Market Opportunities
The global clinical trials outsourcing market has seen significant developments in recent years, particularly with the rise of specialized Contract Research Organizations (CROs) that focus on niche areas of clinical research. These CROs offer innovative approaches and models that are reshaping the market by addressing specific challenges associated with rare and infectious diseases. By providing tailored services and deep expertise, specialized CROs are helping sponsors effectively manage the complexities of clinical trials for these hard-to-treat conditions.
Specialized CROs cater to specific therapeutic areas, clinical trial phases, and patient populations, offering a more customized and efficient experience for sponsors. Their focused expertise in niche diseases allows for more precise patient recruitment and enrollment strategies, which can significantly reduce the time and costs associated with clinical trials. This approach helps mitigate the common challenges of clinical trial inefficiencies, making the process faster and more cost-effective.
Rising Role of Specialized CROs in Clinical Trials
Clinical trials for niche therapeutic areas are particularly challenging due to factors such as limited patient populations, the complexity of treatments, and regulatory hurdles. Even large biopharma companies often face difficulties in conducting clinical trials for these diseases. As a result, there is a growing trend toward outsourcing clinical trials to specialized CROs that have a proven track record in managing such complexities.
According to a 2024 report by Lumis International, sponsors are increasingly turning to specialized CROs to leverage their in-depth knowledge and expertise in niche areas. This trend is driven by the desire to reduce drug time-to-market and improve clinical trial outcomes. By utilizing specialized CROs, biopharma companies can navigate the challenges of rare and niche diseases more effectively, accelerating drug development and ensuring better patient outcomes.
Shift Toward Patient-Centric and Customized Approaches
The growing demand for more patient-centric, customized approaches to clinical trials is also fueling this shift toward specialized CROs. As clinical trials become more focused on specific patient populations and therapeutic areas, sponsors are prioritizing CROs that can offer tailored solutions and deep knowledge of these specialized areas. This patient-focused approach not only improves trial efficiency but also enhances the likelihood of successful outcomes.
As a result, the global clinical trials outsourcing market is expected to expand significantly in several niche therapeutic areas, driven by the increasing reliance on specialized CROs. This trend reflects a broader evolution in the clinical trials industry, as companies seek more effective, efficient, and customized solutions for drug development in rare and complex diseases.
The APAC Emerges as the Fastest-Growing Region in the Clinical Trials Outsourcing Market
In 2023, the Asia-Pacific (APAC) region held the third-largest share of the global clinical trials outsourcing market, with Australia emerging as the primary revenue contributor. APAC continues to be a key destination for clinical trials outsourcing due to several factors, including its large and diverse population, rapidly expanding healthcare infrastructure, and the presence of a significant number of biopharma, medical device companies, and academic institutions.
The region’s appeal is driven by its vast patient pool, cost advantages, and high-quality infrastructure. Clinical trial costs in APAC are generally 30-40% lower than in Western countries, making it an attractive option for US and European companies. Australia, in particular, stands out for its cost-effective clinical trial offerings, supported by government incentives like the Research and Development Tax Incentive (R&DTI), which further encourages the growth of clinical trials.
A favorable regulatory environment and low trial density in APAC also contribute to its growing role in the global market. Regional health authorities are working to improve standardization in clinical trial protocols and enhance interoperability, which is expected to further drive market growth.
In recent years, the region has seen a significant rise in cancer-related clinical trials, and it currently leads the world in the number of such studies. Furthermore, domestic players in APAC are increasingly turning to regional CROs for clinical trial outsourcing, driven by resource constraints and limited capital for in-house R&D.
Overall, APAC combination of cost benefits, regulatory support, and healthcare advancements makes it an increasingly attractive hub for clinical trials, with substantial growth expected in the coming years.
Looking for More Information? Click: https://www.arizton.com/market-reports/bluetooth-speaker-market-analysis-report-2025
Competitive Overview
The global clinical trials outsourcing market is highly competitive and dynamic, with the presence of national, regional, and international players. This market has seen significant growth in recent years, driven by increased research and development (R&D) activities from biotech and pharmaceutical companies, medical device manufacturers, academic institutions, government agencies, and other scientific and clinical research organizations focused on delivering targeted treatments to patients. Key factors such as rising R&D expenditures, government initiatives, and increasing competition within the pharmaceutical industry have fostered the adoption of clinical trials outsourcing. Outsourcing helps reduce costs and time while improving the success rates of new product launches.
Recent Vendors Activities
- In 2021, ICON completed the acquisition of PRA Health Sciences, which will leverage its enhanced operations to transform clinical studies and accelerate the biopharma customers.
- Laboratory Corporation of America Holdings has made 26 acquisitions and 26 investments with spending over $3.3 billion for those acquisitions till 2022. In which the company made significant investments in multiple sectors such as genomics, life sciences, oncology, and more.
- In 2021, Thermo Fisher Scientific acquired a Pharmaceutical Product Development company which is one of the leading market players in the clinical trials outsourcing market. This made the company a significant player in the clinical trials outsourcing segment.
- In 2021, Thermo Fisher Scientific will expand its direct-to-patient services which are designed to support trials and minimize the length of trials and cost which help to boost enrolment and participant rates.
Vendor List
Key Vendors
- Charles River Laboratories
- IQVIA
- ICON plc
- Laboratory Corporation of America Holdings
- Syneos Health
- Thermo Fischer Scientific
- WuXi AppTec
- Paraxel International (MA) Corporation
- Advanced Clinical
- ProteGene (BioAnalytix)
- Curia Global
- AIXIAL (Cmed)
- BioPharma Services
- Cromos Pharma
- CTI Clinical Trial & Consulting
- CRITERIUM
- KCR S.A.
- Medpace
- Medelis
- OCT Clinical
- ProTrials Research
- PROMETRIKA
- Quality Data Services
- QPS Holdings
- Sofpromed
- Veristat
- Worldwide Clinical Trials
- CMIC Holdings
- Vial
- Lumis International
- Novotech
- Clinixir
Segmentation & Forecast
Outsourcing Type
- Functional Service Providers
- Full-service Outsourcing
- Hybrid
Phase
- Phase III
- Phase II
- Phase IV
- Phase I
Indications
- Oncology
- Cardiovascular Disorders (CVD)
- Nervous Systems Diseases
- Infectious Diseases
- Musculoskeletal Diseases
- Gastroenterology Diseases
- Other Indications
End-Users
- Bio & Pharmaceutical Companies
- Academic/Research Institutes & Universities
- Medical Devices Manufacturer
- Other End-user
Geography
North America
- The US
- Canada
Europe
- Germany
- The UK
- France
- Spain
- Italy
APAC
- China
- Japan
- India
- South Korea
- Australia
Latin America
- Brazil
- Mexico
- Argentina
Middle East & Africa
- Turkey
- Egypt
- South Africa
- Saudi Arabia
The Arizton Advisory & Intelligence market research report provides valuable market insights for industry stakeholders, investors, researchers, consultants, and business strategists aiming to gain a thorough understanding of the clinical trials outsourcing market. Request for Free Sample to get a glance of the report now: https://www.arizton.com/market-reports/clinical-trials-outsourcing-market
What Key Findings Will Our Research Analysis Reveal?
Who are the major players in the global clinical trials outsourcing market?
What is the growth rate of the global clinical trials outsourcing market?
What are the factors driving the global clinical trials outsourcing market growth?
How big is the global clinical trials outsourcing market in the global market?
Which region dominates the global clinical trials outsourcing market share?
Looking for Customization According to Your Business Requirement? https://www.arizton.com/customize-report/4588
Other Related Reports that Might be of Your Business Requirement
Global Clinical Trials Software Market – Focused Insights 2024-2029
https://www.arizton.com/market-reports/clinical-trials-software-market-size
Global Virtual Clinical Trials Market – Focused Insights 2024-2029
https://www.arizton.com/market-reports/virtual-clinical-trials-market
Why Arizton?
100% Customer Satisfaction
24×7 availability – we are always there when you need us
200+ Fortune 500 Companies trust Arizton’s report
80% of our reports are exclusive and first in the industry
100% more data and analysis
1500+ reports published till date
Post-Purchase Benefit
- 1hr of free analyst discussion
- 10% off on customization
About Us:
Arizton Advisory and Intelligence is an innovative and quality-driven firm that offers cutting-edge research solutions to clients worldwide. We excel in providing comprehensive market intelligence reports and advisory and consulting services.
We offer comprehensive market research reports on consumer goods & retail technology, automotive and mobility, smart tech, healthcare, life sciences, industrial machinery, chemicals, materials, I.T. and media, logistics, and packaging. These reports contain detailed industry analysis, market size, share, growth drivers, and trend forecasts.
Arizton comprises a team of exuberant and well-experienced analysts who have mastered generating incisive reports. Our specialist analysts possess exemplary skills in market research. We train our team in advanced research practices, techniques, and ethics to outperform in fabricating impregnable research reports.
Media Contact
Company Name: Arizton Advisory & Intelligence
Contact Person: Jessica
Email: Send Email
Phone: +1 3122332770
Country: United States
Website: https://www.arizton.com/market-reports/clinical-trials-outsourcing-market
Press Release Distributed by ABNewswire.com
To view the original version on ABNewswire visit: The Clinical Trials Outsourcing Market to Hit $70 Billion by 2029 – Arizton