Synopsis
Pharmacy Retailing is drugs sold in the retail and bought on the internet, not in the hospital.
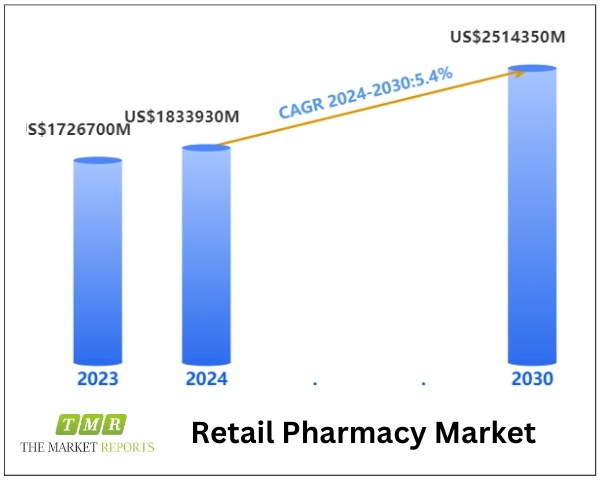
The global Retail Pharmacy Market was valued at US$ 1726700 million in 2023 and is anticipated to reach US$ 2514350 million by 2030, witnessing a CAGR of 5.4% during the forecast period 2024-2030.
The global pharmaceutical market is 1475 billion USD in 2022, growing at a CAGR of 5% during the next six years. The pharmaceutical market includes chemical drugs and biological drugs. For biologics is expected to 381 billion USD in 2022. In comparison, the chemical drug market is estimated to increase from 1005 billion in 2018 to 1094 billion U.S. dollars in 2022. The pharmaceutical market factors such as increasing demand for healthcare, technological advancements, and the rising prevalence of chronic diseases, increase in funding from private & government organizations for development of pharmaceutical manufacturing segments and rise in R&D activities for drugs. However, the industry also faces challenges such as stringent regulations, high costs of research and development, and patent expirations. Companies need to continuously innovate and adapt to these challenges to stay competitive in the market and ensure their products reach patients in need. Additionally, the COVID-19 pandemic has highlighted the importance of vaccine development and supply chain management, further emphasizing the need for pharmaceutical companies to be agile and responsive to emerging public health needs.
This report aims to provide a comprehensive presentation of the global market for Retail Pharmacy, with both quantitative and qualitative analysis, to help readers develop business/growth strategies, assess the market competitive situation, analyze their position in the current marketplace, and make informed business decisions regarding Retail Pharmacy.
Report Scope
The Retail Pharmacy market size, estimations, and forecasts are provided in terms of revenue ($ millions), considering 2023 as the base year, with history and forecast data for the period from 2019 to 2030. This report segments the global Retail Pharmacy market comprehensively. Regional market sizes, concerning products by Type, by Application, and by players, are also provided.
For a more in-depth understanding of the market, the report provides profiles of the competitive landscape, key competitors, and their respective market ranks. The report also discusses technological trends and new product developments.
The report will help the Retail Pharmacy companies, new entrants, and industry chain related companies in this market with information on the revenues, sales volume, and average price for the overall market and the sub-segments across the different segments, by company, by Type, by Application, and by regions.
Request a Sample Copy or Connect for Further Details: https://www.themarketreports.com/report/ask-your-query/1911260
Market Segmentation
By Company
- Walgreens Boots Alliance
- CVS Health
- MedPlus
- Grupo Casa Saba
- Walvax Biotechnology
- UnitedHealth Group
- Dougherty’s Pharmacy
- Medzone
- Tesco
Segment by Type
- Community Pharmacy
- Consult Pharmacy
- Home Care Pharmacy
- Others
Segment by Application
- School
- Community
- Hospital
- Online Retail
- Others
By Region
- North America (United States, Canada and Mexico)
- Europe (Germany, France, United Kingdom, Russia, Italy, and Rest of Europe)
- Asia-Pacific (China, Japan, Korea, India, Southeast Asia, and Australia)
- South America (Brazil, Argentina, Colombia, and Rest of South America)
- Middle East & Africa (Saudi Arabia, UAE, Egypt, South Africa, and Rest of Middle East & Africa)
Chapter Outline
Chapter 1: Introduces the report scope of the report, executive summary of different market segments (by Type, by Application, etc), including the market size of each market segment, future development potential, and so on. It offers a high-level view of the current state of the market and its likely evolution in the short to mid-term, and long term.
Chapter 2: Introduces executive summary of global market size, regional market size, this section also introduces the market dynamics, latest developments of the market, the driving factors and restrictive factors of the market, the challenges and risks faced by companies in the industry, and the analysis of relevant policies in the industry.
Chapter 3: Detailed analysis of Retail Pharmacy companies’ competitive landscape, revenue market share, latest development plan, merger, and acquisition information, etc.
Chapter 4: Provides the analysis of various market segments by Type, covering the market size and development potential of each market segment, to help readers find the blue ocean market in different market segments.
Chapter 5: Provides the analysis of various market segments by Application, covering the market size and development potential of each market segment, to help readers find the blue ocean market in different downstream markets.
Chapter 6, 7, 8, 9, 10: North America, Europe, Asia Pacific, Latin America, Middle East and Africa segment by country. It provides a quantitative analysis of the market size and development potential of each region and its main countries and introduces the market development, future development prospects, market space, and capacity of each country in the world.
Chapter 11: Provides profiles of key players, introducing the basic situation of the main companies in the market in detail, including product sales, revenue, price, gross margin, product introduction, recent development, etc.
Chapter 12: The main points and conclusions of the report.
Read More Related Research Reports:
Pharmacy Automation Market: https://www.themarketreports.com/report/global-pharmacy-automation-market-research-report
Pharmacy Isolators Market: https://www.themarketreports.com/report/global-pharmacy-isolators-market-research-report
Mail Order Pharmacy Market: https://www.themarketreports.com/report/global-mail-order-pharmacy-market-research-report
About US:
At ‘The Market Reports’, we are a trusted market research firm dedicated to empowering businesses with valuable insights and data to drive their success. We offer a wide range of comprehensive market research reports to meet the unique needs of each client. From market analysis and competitive intelligence to consumer behaviour and trend forecasting, we provide the critical information necessary to make informed decisions and stay ahead of the competition. Our goal is to empower our clients with the knowledge they need to drive growth, make strategic investments, and seize new opportunities.
Media Contact
Company Name: The Market Reports
Contact Person: Shirish Gupta
Email: Send Email
Phone: +16314071315
Address:SF-29, Sacred World, Wanawadi
City: Pune
State: Maharastra
Country: India
Website: https://www.themarketreports.com/report/global-retail-pharmacy-market-research-report
Press Release Distributed by ABNewswire.com
To view the original version on ABNewswire visit: Retail Pharmacy Market: US$ 2.51 Trillion, 5.4% CAGR, Driven by Healthcare Demand, Forecast 2024-2030 | Key Players: Walgreens Boots Alliance, CVS Health, MedPlus, Grupo Casa Saba