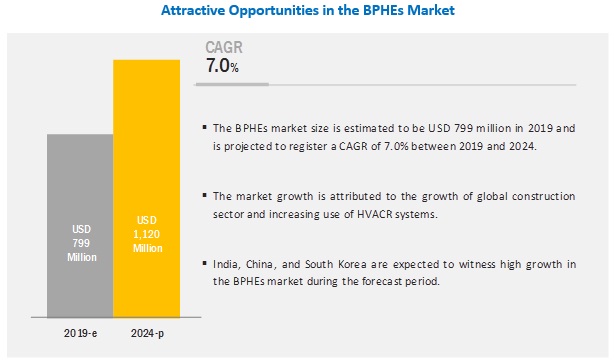
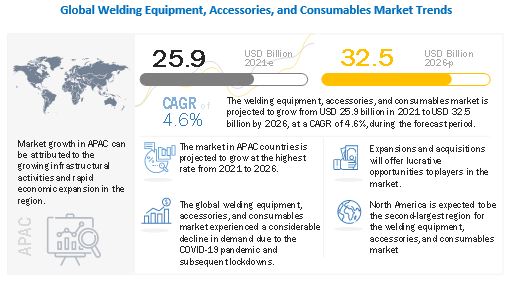
BRISBANE, Calif. – Tempest Therapeutics, Inc. (Nasdaq: TPST), a clinical-stage biopharmaceutical company dedicated to developing novel therapeutics, today announced its participation in the American Society of Clinical Oncology (ASCO) Annual Meeting 2024. The company presented compelling data highlighting the efficacy and safety of its lead candidate, TPST-1120, a first-in-class PPARα antagonist, in the treatment of liver cancer and other malignancies.
At the forefront of oncological innovation, Tempest Therapeutics’ presentation encapsulated the promising results of TPST-1120 in first-line liver cancer treatment. This pivotal study, a part of Tempest’s diverse oncology pipeline, has generated significant interest due to its potential to redefine cancer therapy standards. TPST-1120, through its dual mechanism of targeting tumor cells and modulating the immune system, has shown a favorable impact on overall survival rates and progression-free survival in hepatocellular carcinoma (HCC) patients, as revealed in the data presented at ASCO 2024.
The study findings highlight TPST-1120’s superior performance compared to existing standard of care treatments. Notably, the drug exhibited a remarkable objective response rate (ORR) and a well-tolerated safety profile, offering new hope to liver cancer patients. Its efficacy extends beyond HCC, showing promising results in renal cell carcinoma (RCC) and cholangiocarcinoma (CCA), thereby reinforcing the drug’s versatility and broad-spectrum potential.
Tempest’s commitment to addressing unmet needs in oncology is further evidenced by its robust pipeline of novel clinical programs, including TPST-1495, targeting EP2/EP4 receptors, and an innovative TREX1 inhibitor. These programs, together with TPST-1120, highlight Tempest’s strategic approach to leveraging molecular insights for therapeutic advancements.
Tempest Therapeutics’ CEO commented on the significance of these developments, stating, “Our presentation at the ASCO Annual Meeting 2024 marks a pivotal moment in our journey to transform cancer treatment. The encouraging data from our TPST-1120 study not only validate our scientific approach but also strengthen our resolve to deliver innovative treatments that can significantly improve patient outcomes.”
The company’s unwavering focus on developing therapeutics that combine targeted and immune-mediated mechanisms positions it uniquely in the oncology space. Tempest’s research and development efforts, rooted in scientific rigor and patient-centricity, are set to make substantial contributions to cancer care, offering new avenues for treatment and hope for patients worldwide.
Investors and stakeholders are invited to learn more about Tempest Therapeutics’ groundbreaking research and developments in the field of oncology. For further details, please visit https://www.tempesttx.com/.
About Tempest Therapeutics
Tempest Therapeutics, headquartered in Brisbane, California, is a clinical-stage oncology company. It focuses on advancing small molecules that uniquely combine tumor-targeted and immune-mediated mechanisms. With a diverse portfolio ranging from early research to global clinical studies, Tempest is committed to transforming cancer treatment.
Disclaimer: This press release contains forward-looking statements regarding Tempest Therapeutics’ future plans and expected performance based on current expectations and projections. These statements are subject to risks and uncertainties and actual results may differ materially.
Media Contact
Company Name: Tempest Therapeutics
Contact Person: Media Relations
Email: Send Email
Country: United States
Website: https://www.tempesttx.com/
Press Release Distributed by ABNewswire.com
To view the original version on ABNewswire visit: Tempest Therapeutics Announces Groundbreaking Advances in Liver Cancer Treatment, Showcasing Exceptional Efficacy and Safety Data at ASCO Annual Meeting 2024