AAV One-stop Solution
Process development for triple transfection
Support regulatory filing
AAV vector is widely used delivery vehicle due to its high safety and effectiveness in delivering Gene of Interest (GOI). ProBio is broadening its business in AAV services to cater to the market demand.

One-stop Solution for AAV
ProBio offers services from cell banking, process development, AAV packaging, analytical development, to GMP manufacturing and stability test for AAV vector. ProBio is also ready to prepare reports and documents to support the regulatory filings.
ProBio is also able to produce AAV plasmids to take care of your projects from plasmids to AAV.
Packaging Test
- To evaluate the titer and function of AAV
Demo Run
- Small scale production evaluation
Cell Banking
- MCB/WCB Construction
- Cell Bank Characterization
- Cell Bank Stability Test
Process Development (PD)
- Upstream Process Development
- Downstream Process Development
- Formulation Process Development
Verification Run
- Verification run
- QC and release
Engineering Run
- Full scale in clean room, used for toxicology batch
- QC and release
Analytical Development
- Analytical Method Development
- Analytical Method Qualification
GMP Manufacturing
- GMP Manufacturing
- Quality Control and Release
Stability Test
- Accelerated Stability Test
- Influencing Factor Stability Test
- Long-term Stability Test
Regulatory Support
- Report and document preparation for regulatory filing
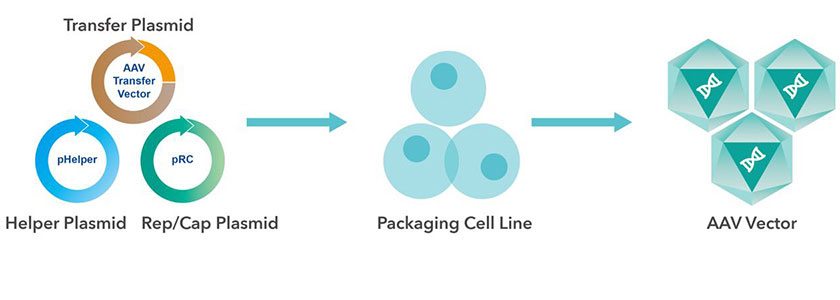
Production of AAV
Among the various ways of producing AAV vector, the most mature, versatile and cost-effective one is triple transient transfection system. Three plasmids, helper plasmid, Rep/Cap plasmid and AAV transfer plasmid, are co-transfected in packaging cell line, such as HEK 293, to manufacture AAV.
Advantages of AAV One-stop Solutions
Professional
Professional team averagely over 10 years of virus production experience
Efficiency
Process development for specific project based on platform experience
Cutting-edge technology
ddPCR for titer measurementAUC for full ratio measurement
Guarantee Delivery
Strict product release criteriaDocument preparation for regulatory filing
Media Contact
Company Name: ProBio CDMO
Email: Send Email
Phone: 1-877-436-7274 (Toll-Free)
Country: United States
Website: https://www.probiocdmo.com
