InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the “Global Diagnostic Contract Manufacturing Market – (By Device (In Vitro Diagnostic Devices (IVD Consumables, IVD Equipment), Diagnostic Imaging Devices (X-ray, CT scan, Ultrasound, MRI, Others), Other Devices),By Service (Device Development & Manufacturing, Quality Management, Packaging & Assembly), By Application (In Vitro Diagnostic Devices (Infectious Diseases, Diabetes, Oncology, Cardiology, Others), Diagnostic Imaging Devices (Cardiology, Neurology, Orthopedics, Oncology, Others), Other Devices (Gastroenterology, Gynecology, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031.”
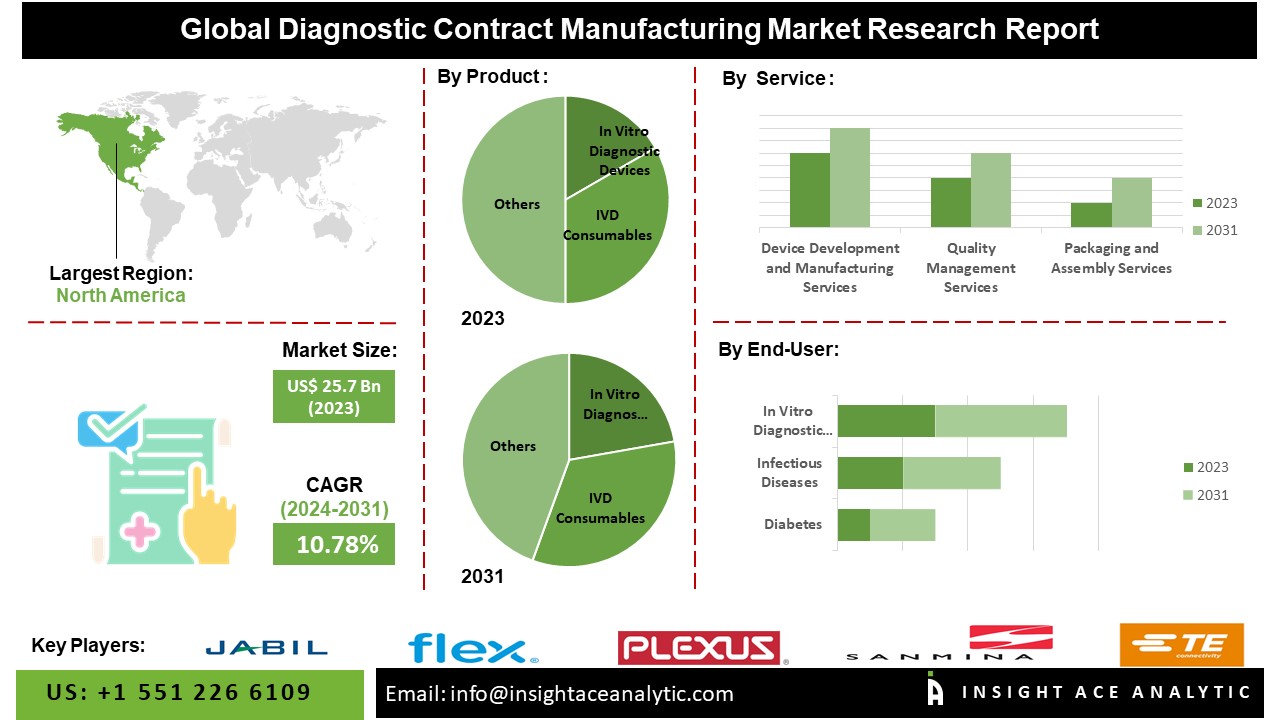
According to the latest research by InsightAce Analytic, the Global Diagnostic Contract Manufacturing Market is valued at US$ 25.7 Billion in 2023, and it is expected to reach US$ 57.9 Billion by 2031, with a CAGR of 10.78% during the forecast period of 2024-2031.
Specialized companies engage in diagnostic contract manufacturing when they produce diagnostic products, components, or assays in accordance with the specifications of other businesses. In order to start the manufacturing process, the business gives the manufacturer all the necessary specifications and, if relevant, the raw materials. The diagnostics industry frequently uses contract manufacturing agreements to outsource diagnostic testing to third parties. This encompasses the whole process, from manufacturing the components to constructing the final product and, occasionally, even packaging it all. It is standard practice in the diagnostics sector to leverage the knowledge and experience of specific manufacturers in order to simplify processes. Several advantages can be gained via IVD contract manufacturing.
Outsourcing to specialist manufacturers allows diagnostic companies to focus on research and development, marketing, and other critical abilities while benefiting from efficient and cost-effective production. This method could lower production expenses, increase operational efficiency, and shorten the time it takes to launch new diagnostic equipment. Businesses can avoid spending a ton of money on infrastructure upgrades by partnering with contract manufacturers who already have a presence in international markets. New developments in in vitro diagnostics are also propelling the industry ahead. However, the high cost of instruments and the maintenance of diagnostic tablets impeded the market’s growth.
Request for Sample Pages: https://www.insightaceanalytic.com/request-sample/2449
List of Prominent Players in the Diagnostic Contract Manufacturing Market:
- Jabil Inc.
- Flex Ltd.
- Plexus Corp
- Sanmina Corporation
- TE Connectivity Ltd.
- Celestica Inc.
- Integer Holdings Corporation
- Nipro Medical Corporation
- Thermo Fisher Scientific
- West Pharmaceutical Services, Inc.
- Benchmark Electronics Inc.
- Kimball Electronics Inc.
- KMC Systems
- Savyon Diagnostics
- Nova Biomedical
- Cenogenic Corporation
- Cone Bioproducts
- Avioq Inc.
- Philips-Medisize Corporation
- Invetech
- Meridian Bioscience, Inc.
- Nolato AB
- Fujirebio
- Sekisui
- Prestige Diagnostics
- Biokit
- Other Market Players
Market Dynamics:
Drivers-
The growing demand for the diagnostic contract manufacturing market is fueled by advancements in outsourcing efficiency and technical convergence. In this age of fast technological development, IVD companies are looking to maximize operational efficiency while reducing costs by outsourcing their manufacturing processes to specialist partners. As a result of the knowledge and efficiency brought by contract manufacturers, IVD companies are free to concentrate on market strategy and innovation. This strategy of collaborative outsourcing not only guarantees cost-effectiveness but also speeds up the creation and distribution of state-of-the-art diagnostic instruments.
Challenges:
The prime challenges are difficulties with personalization, a shortage of competent individuals, and a lack of norms and protocols because of lockdowns and isolation in emerging countries, which are predicted to slow the growth of the diagnostic contract manufacturing market. The complexity of customization can raise production timelines and costs, yet there is a rising need for individualized diagnostic solutions. Because too much personalization hinders production efficiency and makes it difficult for contract manufacturers to satisfy varied market demands, finding the sweet spot between standardized and customized products is critical. The intricate nature of customization and the need to safeguard intellectual property both add layers of complexity to the problems experienced by IVD contract manufacturers. These factors impact consumer demand and the nature of the partnerships that exist within the business. The COVID-19 pandemic broke out, and virus-related disruptions have become increasingly widespread in several industries, including the worldwide in-vitro diagnostic market. Service providers have prioritized sanitizing and reorganizing workspaces as a defence mechanism to increase resilience and decrease security risks. Integrating digital collaboration tools has also been a need for remote customer support. Businesses in the in-vitro diagnostic industry are gradually making a return from the recession by embracing digital technologies that allow them to grow their customer base and increase productivity.
Regional Trends:
The Asia Pacific diagnostic contract manufacturing market is anticipated to register a major market share in revenue. It is projected to grow at a high CAGR in the near future because local businesses have a strong presence in the area, and the infrastructure supporting various industries is expanding. The rising prevalence of long-term health issues, including diabetes, heart disease, and cancer, is also a key factor propelling the IVD industry forward. Besides, North America had a considerable share in the market because of the strong healthcare system and abundance of diagnostic contract manufacturing companies in the area.
Curious About This Latest Version Of The Report? Enquiry Before Buying: https://www.insightaceanalytic.com/report/diagnostic-contract-manufacturing-market/2449
Recent Developments:
- In May 2020, Celestica’s Newmarket Operation has successfully obtained a contract with StarFish Medical Inc. to manufacture ventilators specifically for the Canadian market. StarFish Medical selected Celestica due to its vast expertise in design, supply chain management, ISO 13485 certified production, and dedication to upholding the most stringent quality and regulatory requirements.
- In May 2020, Sanmina Corporation has successfully obtained the Medical Device Single Audit Program certification for its operations in Malaysia, Singapore, and Sweden. The MDSAP accreditation allows for a single audit of the three manufacturing facilities by a recognized third party, ensuring compliance with laws for medical device markets in Australia, Brazil, Canada, Japan, and the US.
Segmentation of Diagnostic Contract Manufacturing Market-
By Device-
- In Vitro Diagnostic Devices
- IVD Consumables
- IVD Equipment
- Diagnostic Imaging Devices
- X-ray
- CT scan
- Ultrasound
- MRI
- Others
- Other Devices
By Service-
- Device Development & Manufacturing
- Quality Management
- Packaging & Assembly
By Application-
- In Vitro Diagnostic Devices
- Infectious Diseases
- Diabetes
- Oncology
- Cardiology
- Others
- Diagnostic Imaging Devices
- Cardiology
- Neurology
- Orthopedics
- Oncology
- Others
- Other Devices
- Gastroenterology
- Gynecology
- Others
By Region-
North America-
- The US
- Canada
- Mexico
Europe-
- Germany
- The UK
- France
- Italy
- Spain
- Rest of Europe
Asia-Pacific-
- China
- Japan
- India
- South Korea
- South East Asia
- Rest of Asia Pacific
Latin America-
- Brazil
- Argentina
- Rest of Latin America
Middle East & Africa-
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
For More Customization @ https://www.insightaceanalytic.com/customisation/2449
Media Contact
Company Name: InsightAce Analytic Pvt. Ltd
Contact Person: Diana D’Souza
Email: Send Email
Country: United States
Website: https://www.insightaceanalytic.com/