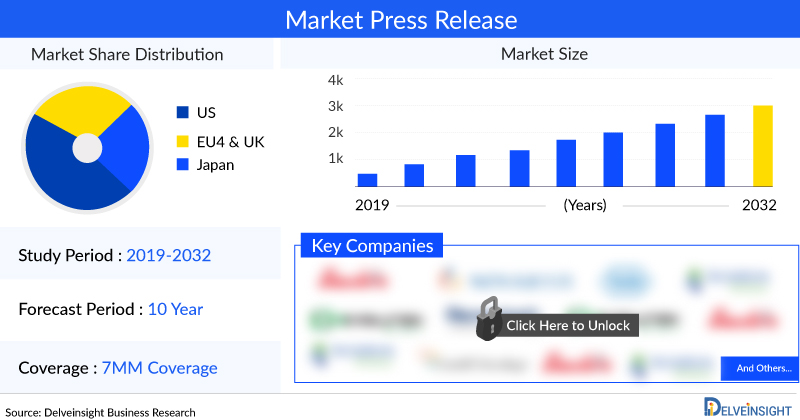
DelveInsight’s “Acute Respiratory Distress Syndrome Market Insights, Epidemiology, and Market Forecast 2034” report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the Acute Respiratory Distress Syndrome market size, share, trends, and growth opportunities in the seven major markets (7MM) (i.e., the United States, EU4 (Germany, Spain, Italy, France), the United Kingdom and Japan).
The Acute Respiratory Distress Syndrome market report covers emerging drugs, current treatment practices, market share of individual therapies, and current & forecasted market size from 2020 to 2034. It also evaluates the current treatment practice/algorithm, key drivers & barriers impacting the market growth, and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
Key highlights from the Acute Respiratory Distress Syndrome Market Report:
-
The acute respiratory distress syndrome market in the 7MM was valued at approximately USD 941 million in 2023 and is projected to grow by 2034. Among the 7MM, the U.S. had the largest market share in 2023, accounting for around USD 666.9 million. This figure is expected to rise further by 2034 due to increasing rates of chronic health conditions and the emergence of new infectious diseases, including novel viral strains.
-
Several factors contribute to the rising incidence of ARDS. Environmental pollutants, such as air pollution and toxins, exacerbate respiratory issues. Additionally, chronic conditions like diabetes, heart disease, and obesity, especially when poorly managed, elevate the risk of developing ARDS.
-
The ARDS market is anticipated to experience steady growth from 2024 to 2034. This growth is driven by an aging population that is more susceptible to long-term illnesses, weakened immune systems, and an increased prevalence of viral and bacterial infections, including pneumonia, influenza, sepsis, and emerging coronavirus strains.
-
Current treatment strategies for ARDS include supportive care, mechanical ventilation, oxygen therapy, sedation, corticosteroids, ACE inhibitors, ARBs, neuromuscular blockers, inhaled vasodilators, and, in some cases, extracorporeal membrane oxygenation (ECMO). Supportive measures such as prone positioning, fluid management, and conservative fluid strategies are also used to optimize patient outcomes.
-
Key Acute Respiratory Distress Syndrome companies are MediciNova, Edesa Biotech, Light Chain Biosciences, Boehringer Ingelheim, Genentech, Windtree Therapeutics, Biomarck Pharmaceuticals, Athersys, Healios, Direct Biologics, Biohaven Pharmaceutical, Arch Biopartners, APEPTICO Forschung und Entwicklung GmbH, Staidson (Beijing) Biopharmaceuticals, Veru, Mesoblast Limited, Avalo Therapeutics, Pluristem Therapeutics, ILTOO Pharma, and others.
-
Expected launch of emerging therapies, such as Traumakine (Faron Pharmaceuticals) BIO-11006 (BioMarck Pharmaceuticals), MultiStem (Athersys), Solnatide (Apeptico Forschung und Entwicklung GmbH), PB1046 (PhaseBio Pharmaceuticals), and others are expected to create a positive impact on the ARDS treatment scenario and ARDS market outlook in the upcoming years.
-
In June 24, 2024, InflaRx N.V. (Nasdaq: IFRX), a biopharmaceutical company pioneering anti-inflammatory therapeutics by targeting the complement system, announced today that GOHIBIC (vilobelimab) has been selected by the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response within the U.S. Department of Health and Human Services, as one of three investigational therapies to be assessed in a Phase 2 clinical platform study exploring potential new options for the treatment of acute respiratory distress syndrome (ARDS).
-
In Dec 2023, the PPD clinical research business of Thermo Fisher Scientific Inc., the world leader in serving science, has been selected by the Biomedical Advanced Research and Development Authority (BARDA) to implement the first BARDA-supported Phase II platform clinical trial to investigate multiple therapeutic options for the treatment of acute respiratory distress syndrome (ARDS). BARDA is part of the Administration for Strategic Preparedness and Response (ASPR) within the U.S. Department of Health and Human Services (HHS).
Read detailed insights on ARDS market outlook 2034 @ https://www.delveinsight.com/report-store/acute-respiratory-distress-syndrome-ards-market
Acute Respiratory Distress Syndrome Overview
Acute respiratory distress syndrome (ARDS) is a rapidly progressive condition that primarily affects critically ill patients. It is marked by the accumulation of fluid in the lungs, which severely impairs breathing and results in low blood oxygen levels. ARDS often arises as a complication of another serious illness or major injury, leading to fluid buildup in the lung’s tiny air sacs and a breakdown of surfactant.
The causes of ARDS are categorized into two main types: direct and indirect lung injuries. Direct lung injuries include conditions such as pneumonia, aspiration, and trauma. Indirect lung injuries involve systemic issues such as pancreatitis, severe infections (sepsis), blood transfusion reactions, burns, and adverse drug reactions. The initial symptoms of ARDS typically include shortness of breath, cough, and fever. Other signs may involve low blood oxygen levels, rapid breathing, and abnormal lung sounds such as clicking, bubbling, or rattling.
Early detection of acute respiratory distress syndrome (ARDS) is crucial for promptly initiating lung-protective ventilation strategies. Despite efforts to refine disease definitions, ARDS is often under-recognized by clinicians, with diagnoses frequently delayed. Reports suggest that up to 40% of ARDS cases are under-diagnosed, with diagnostic rates improving as the disease severity increases. ARDS diagnosis relies on several clinical criteria, none of which are particularly precise. For instance, the Berlin definition has a specificity of only 63% for detecting diffuse alveolar damage (DAD) at autopsy. Currently, no specific biomarker exists for ARDS diagnosis, making this a significant focus of ongoing research.
Chest X-rays (CXR) have relatively low sensitivity and specificity (around 70%) compared to CT scans for diagnosing ARDS. CXR is more effective in identifying diffuse or patchy infiltrates than focal ones. The use of CT scans and other imaging techniques has become increasingly important in diagnosing and managing ARDS. Additionally, lung ultrasound is gaining recognition as a useful bedside tool for facilitating ARDS diagnosis.
Acute Respiratory Distress Syndrome Treatment Market
The treatment of acute respiratory distress syndrome (ARDS) primarily involves supportive care. This includes mechanical ventilation, prevention of stress ulcers and venous thromboembolism, and nutritional support. Effective supportive care for ARDS patients, as with other ICU patients, encompasses early enteral feeding, good glycemic control, prevention of deep venous thrombosis, and prophylaxis against stress ulcers. It is also crucial to identify and treat any underlying infections with antibiotics tailored to culture sensitivities; if cultures are unavailable, antibiotics should target common pathogens relevant to the infection site.
Additional treatments for ARDS patients typically involve supplemental oxygen, prone positioning, the use of neuromuscular blockers (paralytics), fluid management, and positive end-expiratory pressure (PEEP) to help expel fluid from the air sacs. These supportive measures are combined with ongoing treatment of the primary illness or injury. Given that ARDS patients are more susceptible to lung infections, such as bacterial pneumonia, antibiotics are administered to manage these infections. Supportive care may also include intravenous fluids or nutrition as needed.
Acute Respiratory Distress Syndrome Epidemiology Assessment
The epidemiology section provides insights into the historical, current, and forecasted epidemiology trends in the seven major countries (7MM) from 2020 to 2034. It helps to recognize the causes of current and forecasted epidemiology trends by exploring numerous studies and research. The epidemiology section also provides a detailed analysis of diagnosed and prevalent patient pool, future trends, and views of key opinion leaders.
Acute Respiratory Distress Syndrome Epidemiology Insights:
-
In 2023, DelveInsight estimated approximately 862,000 new cases of acute respiratory distress syndrome (ARDS) across the 7MM. This number is expected to rise over the forecast period from 2024 to 2034, largely due to environmental factors such as air pollution and toxin exposure, which exacerbate respiratory diseases.
-
In the U.S., there were about 545,000 new ARDS cases in 2023. This figure is anticipated to grow by 2034 due to the increasing prevalence of chronic conditions like diabetes, heart disease, and obesity.
-
Among the EU4 and the UK, Germany had the highest incidence of ARDS with approximately 157,000 cases in 2023, followed by Italy with around 45,000 cases. Spain had the lowest incidence, with about 21,000 cases.
-
In 2023, the EU4 and the UK reported approximately 44,000 cases of mild ARDS, 132,000 cases of moderate ARDS, and 122,000 cases of severe ARDS.
-
In Japan, there were about 19,000 new ARDS cases in 2023. The primary risk factors included pneumonia (34%), sepsis (29%), trauma (7%), aspiration (10%), pancreatitis (2%), other causes (16%), and unknown factors (2%).
Acute Respiratory Distress Syndrome Emerging Drugs
-
Edesa Biotech/Light Chain Biosciences: EB05 (paridiprubart)
-
Boehringer Ingelheim/ Genentech: Alteplase (Actilyse)
-
BioMarck Pharmaceuticals: BIO-11006
-
Direct Biologics: ExoFlo (DB-001)
Learn How the Acute Respiratory Distress Syndrome Market Will Evolve and Grow by 2034 @ https://www.delveinsight.com/sample-request/acute-respiratory-distress-syndrome-ards-market
Acute Respiratory Distress Syndrome Market/ ARDS Market Outlook, ARDS Treatment Market
Despite significant research efforts, treatment options for acute respiratory distress syndrome (ARDS) are still limited, mainly focusing on supportive care and mechanical ventilation. Current management approaches involve supplemental oxygen, prone positioning, the use of paralytics, fluid management, and positive end-expiratory pressure (PEEP) to help remove fluid from the lungs. Patients often need antibiotics for infections such as bacterial pneumonia and may also require intravenous fluids or nutritional support.
ARDS involves alveolar flooding and pulmonary edema. Studies suggest that β2 agonists might aid in alleviating pulmonary edema by improving sodium transport in alveolar cells. Additionally, accelerating the repair of alveolar epithelial cells is important, with keratinocyte growth factor (KGF) showing potential benefits in both experimental and clinical studies.
Recent advancements include the FDA’s emergency use authorization of Actemra/RoActemra (tocilizumab) for patients requiring supplemental oxygen or mechanical ventilation, and the approval of Pluristem Therapeutics’ Expanded Access Program for PLX-PAD cells in ARDS related to COVID-19. However, these treatments primarily target inflammation rather than directly improving lung function.
Given the substantial unmet need for effective ARDS therapies, several new treatments are being developed and are expected to significantly impact the market. These emerging therapies, such as EB05, BIO-11006, MultiStem, Alteplase, Lucinactant, and DB-001, are projected to be available between 2024 and 2032 and could significantly alter the ARDS treatment landscape.
Leading Players in the Acute Respiratory Distress Syndrome Therapeutics Market Include:
ARDS Companies working in the market are MediciNova, Edesa Biotech, Light Chain Biosciences, Boehringer Ingelheim, Genentech, Windtree Therapeutics, Biomarck Pharmaceuticals, Athersys, Healios, Direct Biologics, Biohaven Pharmaceutical, Arch Biopartners, APEPTICO Forschung und Entwicklung GmbH, Staidson (Beijing) Biopharmaceuticals, Veru, Mesoblast Limited, Avalo Therapeutics, Pluristem Therapeutics, ILTOO Pharma, and others.
Table of Contents
1. Key Insights
2. Executive Summary
3. Acute Respiratory Distress Syndrome Competitive Intelligence Analysis
4. Acute Respiratory Distress Syndrome Market Overview at a Glance
5. Acute Respiratory Distress Syndrome Disease Background and Overview
6. ARDS Patient Journey
7. ARDS Epidemiology and Patient Population (In the US, EU5, and Japan)
8. Acute Respiratory Distress Syndrome Treatment Algorithm, Current Treatment, and Medical Practices
9. ARDS Unmet Needs
10. Key Endpoints of Acute Respiratory Distress Syndrome Treatment
11. Acute Respiratory Distress Syndrome Marketed Products
12. Acute Respiratory Distress Syndrome Emerging Drugs and Latest Therapeutic Advances
13. Acute Respiratory Distress Syndrome Seven Major Market Analysis
14. Attribute Analysis
15. ARDS Market Outlook (In US, EU5, and Japan)
16. ARDS Access and Reimbursement Overview
17. KOL Views on the Acute Respiratory Distress Syndrome Market
18. ARDS Market Drivers
19. ARDS Market Barriers
20. Appendix
21. DelveInsight Capabilities
22. Disclaimer
Trending Reports by DelveInsight:
Adalimumab Biosimilar Market | Arbovirus Infection Market | Artificial Pancreas Device System Market | Dental Equipment Market | Gluten Sensitivity Market | Hypothyroidism Market | Inflammatory Bowel Disease Market | Mayus Kinase Jak Inhibitors Market | Mild Dry Eye Market | Mucopolysaccharidosis Market | Oncolytic Virus Cancer Therapy Market | Pyoderma Gangrenosum Market | Transdermal Drug Delivery Devices Market
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email: Send Email
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/