Cybin Inc., a breakthrough therapy company (NYSE: CYBN) (CBOE CA: CYBN) continues to show that its microcap share price may not be a fair reflection of a company that is helping usher in a transformation in how many mental health conditions are treated. And its method is certainly not more of the same—far from it. This clinical-stage Toronto-based company is developing therapeutics using psychedelic-based compounds, which it believes can offer millions of mental health patients a better and safer approach to treatment.
By advancing its Phase 3 studies in 2024, they may prove that point faster than many think, which would be welcomed news from patients, a developing treatment sector, and investors looking to capitalize on low-priced stocks that are showing the most promising potential to bring these revolutionary treatments to the market.
The better news for Cybin on those fronts is that its robust pipeline positions it well to capitalize on the shifting market dynamics despite the psychedelics industry getting a turbulent jolt from the FDA earlier this month.
Navigating Industry Challenges
That FDA punch was directed at privately held Lykos Therapeutics, which faced near-unanimous rejection from an FDA advisory panel regarding its use of psychedelic compounds in its treatment candidates. As savvy investors would expect, that news took nearly every company in the segment lower in sympathy despite compelling differences that may actually help accelerate product time to market rather than hinder it.
Indeed, Lykos’s setback highlighted the complexities and regulatory hurdles in bringing psychedelic therapies to market. However, the race to market is far from over. Delix Therapeutics CEO Mark Rus noted increased interest and inquiries following Lykos’ advisory committee meeting, indicating a growing recognition of the therapeutic potential and scalability of psychedelic treatments.
Cybin Inc. intends to stay a part of those discussions, a privilege it believes it has earned through its mission and commitment to revolutionize mental health treatment by developing and bringing to patients worldwide innovative psychedelic therapies for conditions such as depression, anxiety, and addiction. Its late-stage candidates are on track to prove that different is good.
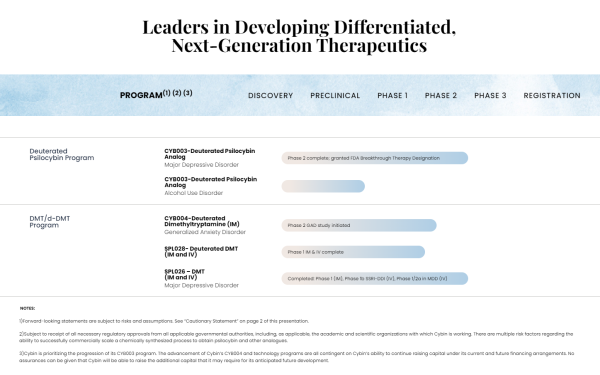
Key Projects and Developments
At the heart of Cybin’s strategy are its flagship projects, CYB003, a Breakthrough Therapy granted by the FDA , CYB004 & CYB005, a portfolio consisting of some of the most advanced and extensive DMT and deuterated DMT (“dDMT”) dataset in the psychedelic drug development. CYB003 is intended to treat major depressive disorder (MDD which is a deuterated psychedelic analog . This innovative approach enhances delivery and efficacy, meeting the increasing demand for efficient and patient-friendly treatments.
CYB004 is currently being evaluated in a Phase 2 study in Generalized Anxiety Disorder (“GAD”) and is believed to be well-positioned to demonstrate a significant therapeutic benefit in the GAD population. Notably, Major Depressive Disorder has significant comorbidity with anxiety: 50% of patients with major depression also have stage 4 anxiety disorder.
Cybin’s internal research has also developed potentially more convenient and patient-friendly dosing methods, including intramuscular (“IM”) dosing, compared to the current intravenous (“IV”) methods used for DMT administration. Another plus, Cybin has also pointed toward its rigorous approach to clinical development, a point CEO Doug Drysdale emphasized in Endpoints News regarding the thorough review process for its upcoming Phase 3 protocol for CYB003, including analyzing and evaluating recent feedback from advisory committee meetings. So far, that’s led to Cybin targeting the larger MDD patient population and limiting the recruitment of refractory patients, which the company says can maximize market potential and streamline clinical trial recruitment.
In the most recent FDA panel decision, Cybin learned in no uncertain terms the importance of including every aspect related to patient safety, even stretching the limits to mitigate potential fringe events not initially included in endpoint considerations. And not just about safety. Cybin also gained insight into satisfying regulatory compliance, particularly in searching for remote signals that could lead to questioning a treatment candidate. In other words, Cybin is making sure it is proactive in expecting the unexpected and, as importantly, collecting data about all adverse events, including abuse-related, underscoring the need for meticulous adherence and regulatory guidelines as well as evaluating potentialities—positive and negative—to demonstrate its dedication to safety and efficacy.
Leveraging Strategic Positioning and Capitalization
Apparently, investors are siding with Cybin’s ability to deliver on its intentions. Before the Lykos advisory committee meeting, mainstream biotech investors recognized Cybin’s potential, leading to a $150 million private placement in March, spearheaded by Deep Track Capital and supported by RA Capital Management and Avidity Partners. This substantial investment shows two things: First, it underscores investor belief in Cybin’s revolutionary vision; Second, it points to a willingness to capitalize on the perceived undervaluation of CYBN stock.
Both are valuable in a competitive landscape. Moreover, bullish backers can fuel pipeline development by allowing Cybin to leverage a strengthening position and capitalize on a receptive and open door of opportunity to prove it can improve healthcare infrastructure. Keep in mind that others are in the game, even Big Pharmaceutical player Johnson & Johnson (NYSE: JNJ), whose candidate, Spravato, targets treating specific mental health indications with a roughly two-hour treatment window and very similar to CYB004, which is for General anxiety use disorder
Cybin’s CYB003 sessions are expected to last four to six hours and adhere to the company’s commitment to optimizing treatment protocols to deliver effective outcomes within practical timeframes. The excellent news is that Cybin, patients, and investors won’t need to wait long for answers about what its treatment candidates can do. Topline results are expected this year, putting potential milestone-induced catalysts in play.
Capitalizing On Open Doors Of Opportunity
There’s a reason the medical industry “practices medicine.” The primary one is that practice makes perfect, or at least gets one closer to that ultimate goal. That fact inspires companies like Cybin to refrain from settling for the status quo or making approved drugs slightly better. It makes them work harder to find alternative ways to better treat medical conditions. In Cybin’s case, they are advancing therapeutic candidates considered unapprovable just a decade ago.
That’s no longer the prevalent thought. And thanks can be given to sub-dollar share-priced companies, like Cybin, that prove innovation is open to those with vision, the ability to innovate, and the willingness to blaze a trail rather than follow those made by others. Cybin has left no doubt about its position- its focus on novel formulations and delivery systems will play a pivotal role in the emerging field of psychedelic medicine.
As mainstream acceptance of psychedelic therapies grows, Cybin’s strategic investments in research and development, coupled with its strong financial foundation, should prove and demonstrate that this company is more than participating in the psychedelic renaissance; it is leading it.
And with strategic partnerships, robust clinical trials, and a relentless focus on delivering better treatments to market that can bring hoped-for relief to millions of patients worldwide, that makes Cybin’s $0.26 share price on Tuesday a value proposition that may be too attractive to ignore. Considering the nine-figure investments by institutional players, many aren’t.
Disclaimers: This presentation has been created by Hawk Point Media Group, Llc. (HPM) and is responsible for the production and distribution of this content. This presentation should be considered and explicitly regarded as sponsored content. Hawk Point Media Group, LLC. has been compensated up to five thousand dollars via wire transfer from IR Agency, Inc. to create this content as part of a more extensive digital marketing program by an unrelated third party to the company. Accordingly, this content may be used and syndicated beyond the channels used by Hawk Point Media, Llc. This disclaimer and the link to the broader disclosures must be part of all reproductions. The compensation received by HPM creates a conflict of interest because the content presented may only provide a favorable viewpoint of the company featured. The contributors do NOT buy and sell securities before and after any article, report, or publication. HPM holds ZERO shares and has never owned stock in Cybin Inc. The information in this video, article, and related newsletters is not intended to be, nor does it constitute, investment advice or recommendations. Hawk Point Media Group, Llc. strongly urges you to conduct a complete and independent investigation of the respective companies and consider all pertinent risks. Readers are advised to review SEC periodic reports: Forms 10-Q, 10K, Form 8-K, insider reports, Forms 3, 4, 5 Schedule 13D. Never take opinions, articles presented, or content provided as the sole reason to invest in any featured company. Investors must always perform their own due diligence before investing in any publicly traded company and understand the risks involved, including losing their entire investment. For the complete disclosure statement, including compensation received, click HERE.
Media Contact
Company Name: Hawk Point Media
Contact Person: Editorial Dept.
Email: Send Email
Country: United States
Website: https://hawkpointmedia.com/

