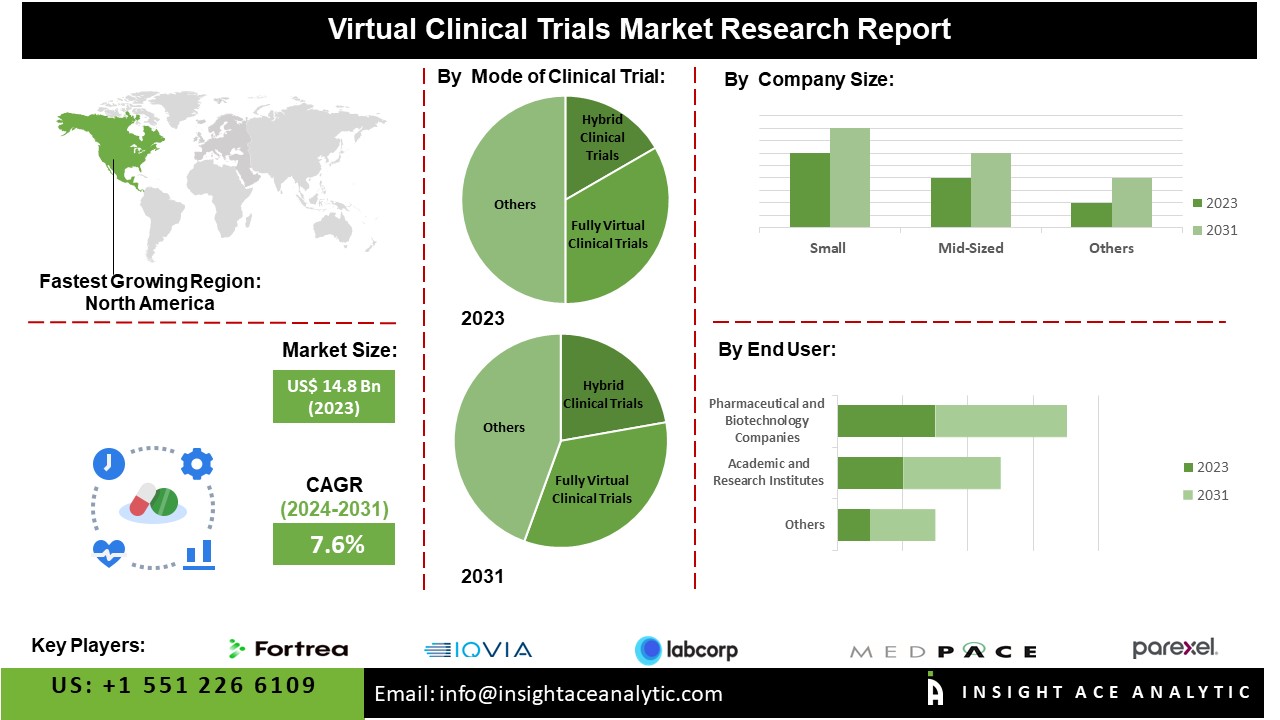
The Global Virtual Clinical Trials Market is estimated to reach over USD 26.3 billion by 2031, exhibiting a CAGR of 7.6% during the forecast period.
The virtual clinical trials market is experiencing significant growth, driven by advancements in digital health technologies, the rise of chronic diseases, and the need for more efficient and cost-effective clinical trial processes. Virtual clinical trials utilize remote monitoring, telemedicine, and digital data collection methods, offering enhanced convenience and accessibility for patients and researchers alike.
This approach addresses several traditional trial challenges, such as geographic limitations, patient recruitment, and retention issues. However, the market also faces challenges, including data security and privacy concerns, regulatory compliance complexities, and disparities in technology access. Despite these hurdles, the COVID-19 pandemic has accelerated the adoption of virtual trials, highlighting their potential to maintain continuity in clinical research during global disruptions. Key market players are investing in advanced digital platforms and forging strategic partnerships to enhance their service offerings and expand their reach.
Overall, the virtual clinical trials market is poised for continued expansion, supported by technological innovations and an increasing focus on patient-centric research models.
Request Free Report Sample Pages: https://www.insightaceanalytic.com/request-sample/2509
List of Prominent Players in the Virtual Clinical Trials Market:
- ICON, plc
- Parexel International Corporation
- IQVIA
- Covance
- PRA Health Sciences
- LEO Innovation Lab
- Medidata
- Oracle
- CRF Health
- Clinical Ink
- Medable, Inc.
- Signing Health
- Halo Health Systems
- Croprime
Market Dynamics:
Drivers-
The increasing of chronic diseases is a prime driver of the virtual clinical trials market, as it necessitates efficient, cost-effective, and patient-centric research methods to expedite drug development and improve patient outcomes, leveraging remote technologies to enhance trial accessibility and data collection. The spike in virtual trials is also driving the virtual clinical trials market by demonstrating their efficiency, reduced costs, and enhanced patient recruitment and retention. This surge reflects a shift towards leveraging digital technologies for more flexible, accessible, and streamlined clinical research processes.
The convenience provided by virtual trials over traditional methods is a key driver of the virtual clinical trials market, offering benefits like remote participation, reduced travel, real-time data collection, and improved patient compliance. This ease enhances patient engagement and accelerates the drug development process.
Challenges:
The prime challenge faced by the market is data security and patient privacy, which includes ensuring the protection of sensitive patient data against breaches and cyberattacks. Regulatory Compliance is another hindrance to growth, including navigating varied and evolving regulatory requirements across different regions. Technological access involves disparities in digital devices and reliable internet among participants. Managing the distribution and use of at-home diagnostic tools and medications, including cost-related ones, is another challenge faced by the Virtual Clinical Trials Market.
Regional Trends:
The North America Virtual Clinical Trials Market is expected to register a major market share in terms of revenue as well as is estimated to grow at a high CAGR in the near future. Growing population, rapid urbanization, and increasing industrialization are factors expected to increase the growth of the Virtual Clinical Trials Market in the region. Besides, Asia-Pacific had a substantial share in the market due to the adoption of new strategies by the major players in the Virtual Clinical Trials Market. Moreover, the presence of key market players along with increasing collaboration among major players for market penetration in the region, provides the opportunity for growth of the Market.
Curious about this latest version of the report? @ https://www.insightaceanalytic.com/enquiry-before-buying/2509
Recent Developments:
- In July 2023, Signant Health completed the acquisition of DSG, strategically augmenting its eClinical solution suite for both traditional and decentralized clinical trials. By integrating DSG’s unified platform, the acquisition facilitated the development of a comprehensive trial ecosystem equipped with advanced software, analytics, and logistics solutions. This enabled seamless study conduct and data generation across all modalities, thereby accomplishing the goal of fully digitalizing clinical trials.
- In June 2023, Medable Inc. unveiled a comprehensive toolkit tailored for Institutional Review Boards (IRBs)/Ethics Committees (ECs), designed to establish standardized ethics review procedures for decentralized clinical trials (DCTs). The implementation of this toolkit successfully simplified, streamlined, and accelerated the IRB/EC process, playing a pivotal role in fostering enhanced efficiency and patient-centeredness in the execution of DCTs.
- In October 2022, Oracle and ObvioHealth entered into a strategic collaboration to integrate diverse data sets into virtual/decentralized clinical trials in the Asia Pacific region. This initiative is expected to allow the quick collection, integration, and analysis of multi-source data collected from labs, devices, patients, and sites.
Segmentation of Virtual Clinical Trials Market-
By Mode of Clinical Trial
- Hybrid Clinical Trial
- Fully Virtual Clinical Trial
By Study Type
- Interventional
- Observational
- Expanded Access
By Type of Therapeutic Area
- Cardiovascular Disorders
- Infectious Diseases
- Metabolic Disorders
- Neurological Disorders
- Oncological Disorders
- Respiratory Disorders
- Other Disorders
By Clinical Trial Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Company Size
- Small
- Mid-sized
- Large
By End Users
- Pharmaceutical and Biotechnology Companies
- Academic and Research Institutes
- Medical Device Industries
- Other End Users
By Region-
North America-
- The US
- Canada
- Mexico
Europe-
- Germany
- The UK
- France
- Italy
- Spain
- Rest of Europe
Asia-Pacific-
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
Latin America-
- Brazil
- Argentina
- Rest of Latin America
Middle East & Africa-
- GCC Countries
- South Africa
- Rest of Middle East and Africa
Media Contact
Company Name: InsightAce Analytic Pvt. Ltd
Contact Person: Diana D’Souza
Email: Send Email
Country: United States
Website: https://www.insightaceanalytic.com/