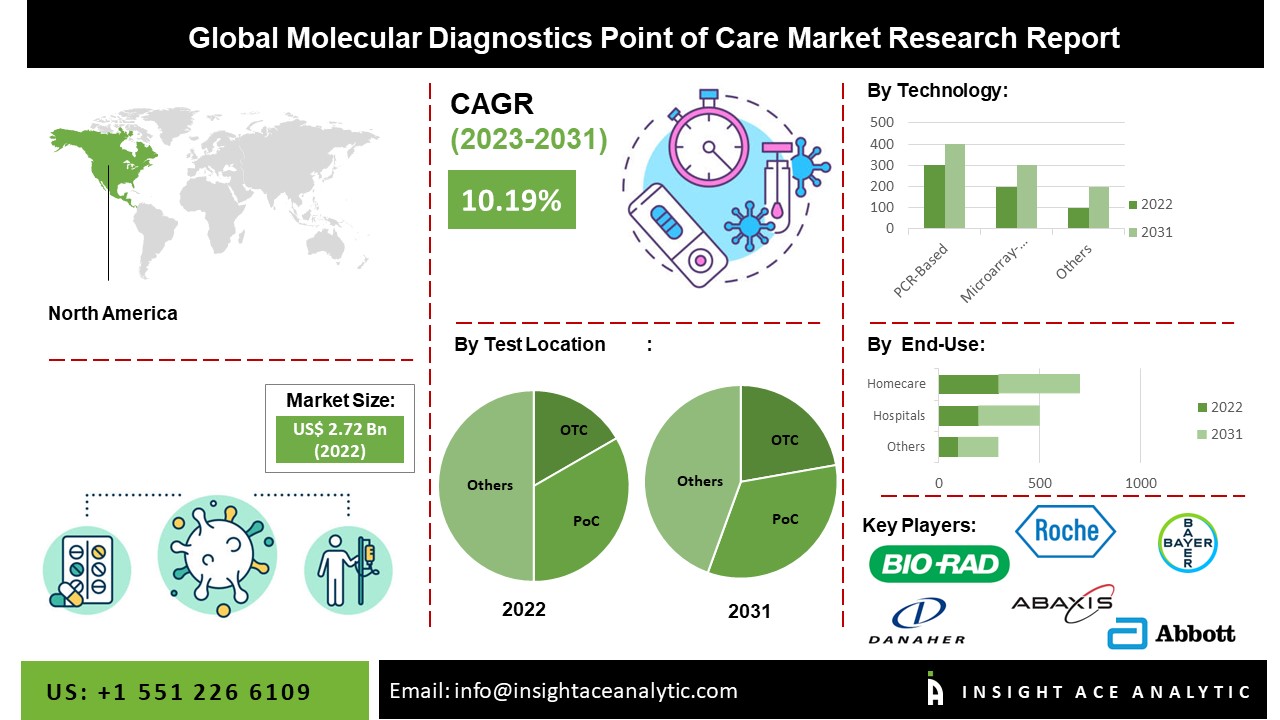
“Molecular Diagnostics Point of Care Market” in terms of revenue was estimated to be worth $2.95 billion in 2023 and is poised to reach $6.42 billion by 2031, growing at a CAGR of 10.38% from 2024 to 2031 according to a new report by InsightAce Analytic.
Get Free Sample Report @ https://www.insightaceanalytic.com/request-sample/1423
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global Molecular Diagnostics Point of Care market are:
- Rising Prevalence of Infectious Diseases
- Investments in Healthcare Facilities
- Integration with Digital Health Solutions
The following are the primary obstacles to the Molecular Diagnostics Point of Care market’s expansion:
- Cost Constraints
- Limited Imbursement Policies
- Infrastructure and Connectivity Issues
Future expansion opportunities for the global Molecular Diagnostics Point of Care market include:
- Market Penetration in Emerging Markets
- Regulatory Compliance
- Point-of-Care Testing in Clinical Trials
Market Analysis:
The rise in the prevalence of infectious diseases and chronic illnesses like cancer is expected to drive the market’s growth. This prevalence has led to increased demand for novel diagnostic methods, further leading to progress in technological advancements and pharmacogenomics. Furthermore, technological advancements and demand for personalized medicine are balanced against restraints like cost constraints and regulatory hurdles.
List of Prominent Players in the Molecular Diagnostics Point of Care Market:
- Abbott Laboratories
- Bayer Healthcare
- Roche Diagnostics
- OraSure Technologies Inc.
- Nipro Diagnostics
- Bio-Rad Laboratories
- Danaher Corporation
- BioMerieux, Dako
- Abaxis Inc.
Molecular Diagnostics Point of Care Market Report Scope:
|
Report Attribute |
Specifications |
|
Market size value in 2023 |
USD 2.95 billion |
|
Revenue forecast in 2031 |
USD 6.42 billion |
|
Growth rate CAGR |
CAGR of 10.38% from 2024 to 2031 |
|
Quantitative units |
Representation of revenue in US$ Million, and CAGR from 2024 to 2031 |
|
Historic Year |
2019 to 2023 |
|
Forecast Year |
2024-2031 |
|
Report coverage |
The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
|
Segments covered |
By Test Location, Technology, Application, End-Use |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
Recent Developments:
- In February 2022, Sense Biodetection entered into a strategic agreement with Una Health (Una) for the United Kingdom distribution of the Veros Covid-19 test.
- In December 2021, FIND invested USD 21 million in Biomeme, Bioneer, Qlife, and SD Biosensor to accelerate the development, manufacturing, and launch of affordable point-of-care molecular diagnostic platforms that can detect multiple pathogens that cause diseases including COVID-19.
- In Oct 2018, QIAGEN negotiated with STAT-Dx to enhance its portfolio in testing solutions for next-gen PCR systems.
Curious about this latest version of the report? @ https://www.insightaceanalytic.com/enquiry-before-buying/1423
Molecular Diagnostics Point of Care Market Dynamics:
Market Drivers: Rising Prevalence of Infectious Diseases
The increasing incidence of infectious diseases worldwide, including COVID-19, influenza, HIV/AIDS, tuberculosis, and sexually transmitted infections, drives the demand for rapid and accurate diagnostic tests at the point of care. Molecular POC tests offer quick results, enabling prompt treatment and containment of infectious outbreaks. Furthermore, the frequent occurrence of infectious diseases and cancer in both developed and developing regions is set to favor the expansion of the point-of-care molecular diagnostics market. Moreover, the growing inclination towards preventive medicine further contributes to the anticipated rise in demand for point-of-care molecular diagnostics throughout the forecast period.
Challenges: Limited Reimbursement Policies
Inadequate reimbursement coverage for molecular POC tests by public and private payers can hinder market growth. Reimbursement policies may not fully reflect the value of POC testing in terms of improved patient outcomes, reduced healthcare costs, and resource utilization, discouraging healthcare providers from investing in these technologies. One of the significant challenges faced by diagnostic companies is obtaining payment from Medicare and private health insurers for their tests. In the United States, Medicare revised its reimbursement mechanism for certain in vitro diagnostic (IVD) tests, including point-of-care molecular tests 2018. Furthermore, Medicare currently does not provide coverage for genetic testing in individuals without a personal history of cancer, as indicated by the American Society of Clinical Oncology. These factors are anticipated to have an adverse impact on the point-of-care molecular testing market.
North America Is Expected To Grow With the Highest CAGR during the Forecast Period
The North America Molecular Diagnostics Point of Care Market is likely to register a significant revenue share and to develop at a rapid CAGR in the near future. This is due to the increasing incidence of infectious diseases, increasing Clinical Laboratory Improvement Amendments product approvals, and expanding legislative measures that are driving this market’s expansion in North America. The ongoing efforts of major market players to strengthen their position are a significant contributing factor. Several clinical trials will contribute to market growth throughout the projection period due to the diversity and precision of contemporary molecular tests. Furthermore, , this region is expected to continue growing as the demand for rapid and accurate diagnostic solutions increases, supported by technological advancements, regulatory initiatives, and evolving healthcare delivery models.
Segmentation of Molecular Diagnostics Point of Care Market-
By Test Location-
- Over-the-counter (OTC)
- Point of Care (POC)
By Technology-
- PCR Based
- Genetic Sequencing Based
- Hybridization-Based
- Microarray-Based
- Nucleic Acid Amplification Testing (NAAT)
By Applications-
- Infectious Disease Testing
- Oncology
- Hematology
- Prenatal Testing
- Endocrinology
- Others
By End Users-
- Decentralized Labs
- Hospitals & Clinics
- Home Care Settings
- Assisted Living Healthcare Facilities
- Emergency Medical Services (EMS)
- Others
By Region-
North America-
- The US
- Canada
- Mexico
Europe-
- Germany
- The UK
- France
- Italy
- Spain
- Rest of Europe
Asia-Pacific-
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
Latin America-
- Brazil
- Argentina
- Rest of Latin America
Middle East & Africa-
- GCC Countries
- South Africa
- Rest of Middle East and Africa
For More Customization @ https://www.insightaceanalytic.com/customisation/1423
Media Contact
Company Name: InsightAce Analytic Pvt. Ltd
Contact Person: Diana D’Souza
Email: Send Email
Country: United States
Website: https://www.insightaceanalytic.com/