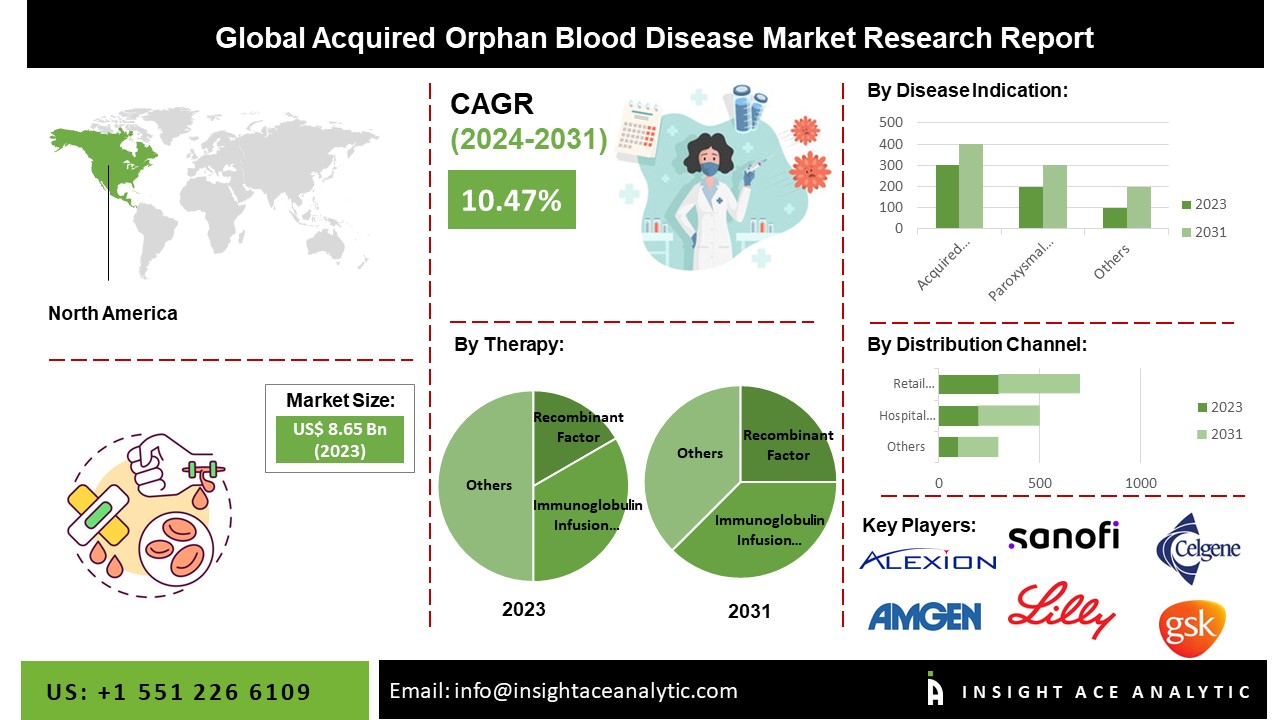
According to a new report by InsightAce Analytic, the “Acquired Orphan Blood Disease Market” in terms of revenue was estimated to be worth $8.65 billion in 2023 and is poised to reach $18.93 billion by 2031, growing at a CAGR of 10.47% from 2024 to 2031.
Buy Now This Report @ https://www.insightaceanalytic.com/buy-report/1456
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global Acquired Orphan Blood Disease Market are:
- Increased Research And Development Initiatives
- Advancements In Personalized Medicine
- Rising Awareness About Rare Diseases
The following are the primary obstacles to the Acquired Orphan Blood Disease Market’s expansion:
- Complex Regulatory Pathways
- Limited Patient Population
- High Development Costs
Future expansion opportunities for the global Acquired Orphan Blood Disease Market include:
- Research And Development Funding
- Orphan Drug Designation Incentives
- Advancements In Precision Medicine
Market Analysis:
The pharmaceutical and healthcare industries that are dedicated to creating and offering therapies for uncommon and orphan blood illnesses that are acquired rather than congenital are referred to as the “Acquired Orphan Blood Disease Market.” Because these disorders are uncommon, they often only impact a small number of people and necessitate specialized therapies.
List of Prominent Players in the Acquired Orphan Blood Disease Market:
- Alexion Pharmaceuticals, Inc.,
- Amgen, Inc.,
- Celgene Corporation,
- Eli Lilly and Company,
- Sanofi S.A.,
- GlaxoSmithKline plc.
- Cyclacel Pharmaceuticals, Inc.,
- Onconova Therapeutics, Inc.,
- Incyte Corporation a
- CTI BioPharma Corp.
Acquired Orphan Blood Disease Market Report Scope:
|
Report Attribute |
Specifications |
|
Market size value in 2023 |
USD 8.65 billion |
|
Revenue forecast in 2031 |
USD 18.93 billion |
|
Growth rate CAGR |
CAGR of 10.47% from 2024 to 2031 |
|
Quantitative units |
Representation of revenue in US$ Million, and CAGR from 2024 to 2031 |
|
Historic Year |
2019 to 2023 |
|
Forecast Year |
2024-2031 |
|
Report coverage |
The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
|
Segments covered |
By Therapy, Disease Indication, Distribution Channel |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
Recent Developments:
- In February 2019, the U.S. FDA approved Cablivi (caplacizumab-yhdp) injection, the first therapy indicated in combination with plasma exchange and immunosuppressive therapy for the treatment of thrombotic thrombocytopenic purpura (aTTP).
- In March 2018, ADDMEDICA received U.S. FDA approval for the Orphan Drug Siklos, the first and sole hydroxyurea-based treatment for pediatric patients with sickle cell anemia.
Curious about this latest version of the report? @ https://www.insightaceanalytic.com/enquiry-before-buying/1456
Acquired Orphan Blood Disease Market Dynamics:
Market Drivers: Increased Research And Development Initiatives
The Acquired Orphan Blood Disease Market is witnessing a surge due to increased research and development (R&D) initiatives. As pharmaceutical and biotechnology companies invest more in understanding and addressing rare blood disorders, it leads to the development of innovative therapies and treatments. Heightened R&D efforts contribute to a deeper understanding of the molecular mechanisms underlying orphan blood diseases, fostering the discovery of targeted and effective interventions. Moreover, such initiatives attract collaborations and partnerships, promoting knowledge exchange and accelerating the pace of drug development. The emphasis on R&D reflects a commitment to addressing unmet medical needs, driving advancements in the Acquired Orphan Blood Disease Market, and offering hope for improved outcomes for patients with rare blood disorders.
Challenges: High Development Costs
The Acquired Orphan Blood Disease Market faces challenges primarily attributed to high development costs. The rarity of orphan blood diseases results in limited patient populations for clinical trials, leading to increased per-patient trial costs. Moreover, the intricate nature of these diseases demands extensive research, sophisticated diagnostic tools, and specialized therapies, further elevating developmental expenses. The smaller market size and potential limited commercial returns pose financial hurdles for pharmaceutical companies investing in research and development for these conditions. Striking a balance between affordability and innovative treatments becomes challenging, hindering the progression of therapeutic advancements. Collaboration among stakeholders, regulatory support, and innovative funding models are crucial to overcoming the financial barriers and ensuring continued progress in addressing acquired orphan blood diseases.
North America Is Expected To Grow With The Highest CAGR During The Forecast Period
In North America, the Acquired Orphan Blood Disease Market is gaining traction owing to advanced healthcare infrastructure, robust research and development activities, and a favorable regulatory landscape. The region, particularly the United States, plays a pivotal role in the market, driven by a high prevalence of orphan blood diseases and a well-established healthcare system. The presence of key pharmaceutical and biotechnology companies, along with increasing awareness among healthcare professionals and patients, contributes to market growth. Additionally, government initiatives and supportive reimbursement policies enhance the accessibility of orphan blood disease treatments. The North American Acquired Orphan Blood Disease Market showcases a dynamic landscape characterized by innovation, collaborations, and a commitment to addressing the unmet medical needs of patients.
Segmentation of Acquired Orphan Blood Disease Market –
By Therapy-
- Recombinant Factor
- Immunoglobulin Infusion Therapy
- Activated Prothrombin Complex Concentrate
- Thrombopoietin Receptor Agonists
- Others
By Disease-
- Acquired Agranulocytosis
- Acquired Hemophilia
- Acquired Von Willebrand Syndrome
- Paroxysmal Nocturnal Hemoglobinuria (PNH)
- Myelodysplastic Syndrome
- Other
By Distribution Channel-
- Hospital Pharmacy
- Retail Pharmacy
- other
By Region-
North America-
- The US
- Canada
- Mexico
Europe-
- Germany
- The UK
- France
- Italy
- Spain
- Rest of Europe
Asia-Pacific-
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
Latin America-
- Brazil
- Argentina
- Rest of Latin America
Middle East & Africa-
- GCC Countries
- South Africa
- Rest of Middle East and Africa
For More Customization @ https://www.insightaceanalytic.com/customisation/1456
Media Contact
Company Name: InsightAce Analytic Pvt. Ltd
Contact Person: Diana D’Souza
Email: Send Email
Country: United States
Website: https://www.insightaceanalytic.com/