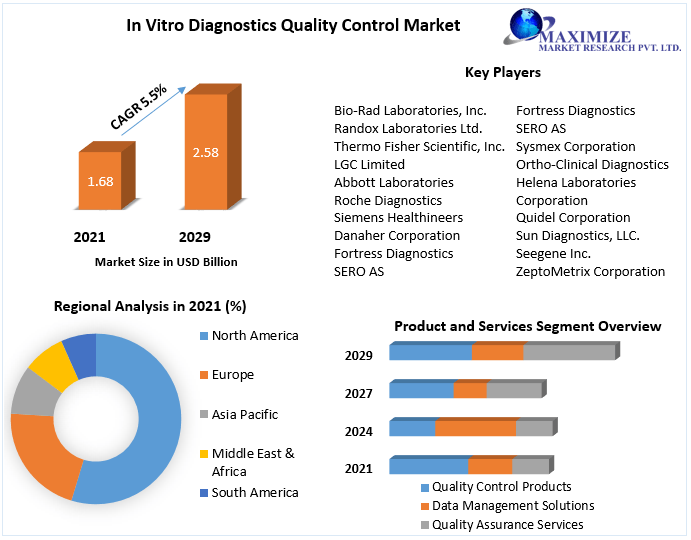
Pune, 24, Nov 2022: A research on the In Vitro Diagnostics (IVD) Quality Control Market offered by Maximize Market Research, a leading healthcare consulting organization. According to the MMR analysis, the In Vitro Diagnostics (IVD) Quality Control Market size was valued at USD 1.68 Bn. in 2021, and the total In Vitro Diagnostics (IVD) Quality Control revenue is expected to grow at 5.5% from 2022 to 2029, reaching nearly USD 2.58 Bn. The report investigates industry trends, major sectors, viable investment regions, demand and supply chains, regional environments, and competitive situations in depth. The research is a valuable resource for important market players, new entrants, investors, and stakeholders who are developing plans and working to improve the reputation of their brands.
Request Free Sample Copy (To Understand the Complete Structure of this Report [Summary + TOC]) @all https://www.maximizemarketresearch.com/request-sample/161874
In Vitro Diagnostics (IVD) Quality Control Market Scope and Research Methodology
The In Vitro Diagnostics (IVD) Quality Control Market report includes data on the Market’s dynamics, such as drivers, restraints, opportunities, trends, and challenges. Regional market analysis is also provided industrial statistics as a resource for market entrants and followers in developing pricing and marketing strategies to manage their operations. The In Vitro Diagnostics (IVD) Quality Control Market study is an in-depth examination of key trends and current market dynamics in the In Vitro Diagnostics (IVD) Quality Control Industry, assisting customers in identifying business opportunities and building market strategies.
The market is segmented by Product and Service, Technology, End User, and region. The report investigates major players’ advanced products and satisfying services, revenue growth, economic position in the global market, investment plan, growth strategy, and geographical presence. Recent acquisitions and mergers, as well as business strategic orientations and information on cooperative partnerships, provide a realistic picture of the In Vitro Diagnostics (IVD) Quality Control Market’s competitive landscape.
The bottom-up strategy is used to estimate the market. Data and statistics were collected on a wide sample utilizing both primary and secondary research methodologies to reach high accuracy. Secondary data was acquired from a carefully selected group of sources to verify that the results were consistent. This includes official databases from different organizations and government websites, industry magazines, newspapers, large corporate annual reports, manufacturer and supplier publications, and paid databases.
To collect primary data, surveys, questionnaire administration, and phone interviews with industry experts, market leaders, entrepreneurs, and marketing professionals are conducted. SWOT analysis was used to examine the strengths and weaknesses of key firms, while PORTER and PESTLE were used to study various economic concerns. Finally, the research provides a comprehensive analysis of the In Vitro Diagnostics (IVD) Quality Control Market.
In Vitro Diagnostics (IVD) Quality Control Market Overview
In Vitro Diagnostics (IVD) is a critical and constantly expanding part of the overall healthcare system that benefits patients, medical professionals, and the industry while also enhancing population well-being. IVD quality controls are samples/materials used to validate the dependability of the IVD testing system in order to ensure the accuracy of test results and to analyze the impact of factors such as environmental conditions and operator performance on test results.
In Vitro Diagnostics (IVD) Quality Control Market Dynamics
The key drivers driving market growth include an increase in the global prevalence of infectious diseases, HIV, and cancer, all of which need improved diagnostic technologies for efficient treatment and quality controls to monitor their performance. In 2019, 24.5 million HIV patients globally acquired antiretroviral treatment, according to the Joint United Nations Programme on HIV and AIDS (UNAIDS). Similarly, increased government participation in managing infectious disease outbreaks, as well as an increased need for quality evaluation support and rapid diagnosis systems, are expected to contribute to global market growth over the forecast period.
The presence of supporting regulatory agencies and an increasing number of accredited clinical laboratories throughout the world are likely to be important drivers of market expansion during the forecast period. Installing a QC procedure in a clinical laboratory, on the other hand, is an expensive endeavor. Laboratories must also retain dedicated personnel on standby to manage the QC system. Furthermore, regardless of the number of tests done, QC processes incur the same costs. As a result, adopting quality control methods is prohibitively expensive for clinical laboratories that perform a limited number of diagnostic tests. This, along with cost constraints in many hospitals and laboratories in both developed and developing nations, is likely to reduce the use of quality control measures.
In Vitro Diagnostics (IVD) Quality Control Market Regional Insights
North America held the greatest market share in 2021 and is expected to lead the In Vitro Diagnostics quality controls market with a CAGR of around 5.1% during the forecast period. The market in the area is likely to be driven by the region’s high rate of adoption of sophisticated infrastructure, a growing number of diagnostic facilities, and renowned clinical laboratories.
Because of the rising frequency of chronic illnesses in 2021, the United States led the market in North America. According to a 2020 U.S. Department of Health and Human Services report, approximately 37,832 people in the United States were diagnosed with HIV in 2018, and 38,000 new HIV infections occur each year in the United States and 6 dependent areas, resulting in the rapid spread of various infectious diseases such as meningitis and urinary tract infections, fueling the market and contributing to its outstanding share of global market revenue during the forecast period.
During the projected period, the Asia-Pacific market is estimated to increase at a CAGR of 4.2%. The rising prevalence of infectious diseases, such as the COVID-19 outbreak, has increased R&D investment, bolstering regional market trends. The demand for accurate in vitro diagnostics solutions is growing in response to the pressing need to address current healthcare issues. Regional governments are taking a variety of steps to encourage the indigenous development of better testing equipment.
In Vitro Diagnostics (IVD) Quality Control Market Segmentation
By Product And Service
- Quality Control Products
- Data Management Solutions
- Quality Assurance Services
By Technology
- Immunochemistry
- Clinical Chemistry
- Molecular Diagnostics
- Microbiology
- Hematology
- Coagulation/ Hemostasis
- Other Technologies
By End User
- Hospitals
- Clinical Laboratories
- Academic and Research Institutes
- Home-care
- Other End users
In Vitro Diagnostics (IVD) Quality Control Market Key Competitors:
- Bio-Rad Laboratories, Inc.
- Randox Laboratories Ltd.
- Thermo Fisher Scientific, Inc.
- LGC Limited
- Abbott Laboratories
- Roche Diagnostics
- Siemens Healthineers
- Danaher Corporation
- Fortress Diagnostics
- SERO AS
- Sysmex Corporation
- Ortho-Clinical Diagnostics
- Helena Laboratories Corporation
- Quidel Corporation
- Sun Diagnostics, LLC.
- Seegene Inc.
- ZeptoMetrix Corporation
- Qnostics
- Bio-Techne Corporation
- Microbiologics
- Microbix Biosystems
- Streck, Inc.
- Alpha-Tec Systems
- Maine Molecular Quality Controls, Inc.
- Grifols, S.A.
To remain ‘ahead’ of your competitors, request for a sample @ https://www.maximizemarketresearch.com/request-sample/161874
Key questions answered in the In Vitro Diagnostics (IVD) Quality Control Market are:
- What are In Vitro Diagnostics (IVD) Quality Controls?
- What is the growth rate of the In Vitro Diagnostics (IVD) Quality Control Market for the next five years?
- What is the nature of competition in the In Vitro Diagnostics (IVD) Quality Control industry in developed economies and developing economies?
- Who are the key players in the In Vitro Diagnostics (IVD) Quality Control Market?
- Who are the market leaders in In Vitro Diagnostics (IVD) Quality Control in Europe
- Who are the market leaders in In Vitro Diagnostics (IVD) Quality Control in USA and Canada
- Who are the market leaders in In Vitro Diagnostics (IVD) Quality Control in India, China, Japan, and South Korea?
- What are the factors affecting growth in the In Vitro Diagnostics (IVD) Quality Control Market?
- Who held the largest market share in the In Vitro Diagnostics (IVD) Quality Control Market?
- What are the factors for the growth of the Asia-Pacific region in the In Vitro Diagnostics (IVD) Quality Control Market?
Key Offerings:
- Market Share, Size & Forecast by Revenue | 2022−2029
- Market Dynamics – Growth Drivers, Restraints, Investment Opportunities, and Key Trends
- Market Segmentation – A detailed analysis Segment and Region
- Competitive Landscape – Top Key Vendors and Other Prominent Vendors
Maximize Market Research is leading healthcare research firm, has also published the following reports:
Alzheimer’s Therapeutics Market: Alzheimer’s Therapeutics Market size was valued at USD 4.5 Bn. in 2021 and the total Alzheimer’s Therapeutics revenue is expected to grow at a CAGR of 19.3% from 2022 to 2029, reaching nearly USD 18.46 Bn. The increasing use of biomarkers in Alzheimer’s diagnosis and medication development, as well as the rising incidence of Alzheimer’s disease globally, are the primary drivers driving the growth of the Alzheimer’s therapeutics market.
Sarcoma Drugs Market: Sarcoma Drugs Market size was valued at USD 759.2 Mn. in 2021 and the total Sarcoma Drugs revenue is expected to grow by 23 % from 2022 to 2029, reaching nearly USD 1405.3 Mn. Immunotherapy has become an important part of treating certain types of cancer in recent years. New immunotherapy treatments are being tested and approved at a rapid pace, as are new ways of working with the immune system. Immunotherapy works better for various cancers than others. It is used alone for some of these cancers, but it appears to work better when combined with other types of treatment for others.
About Maximize Market Research:
Maximize Market Research is a multifaceted market research and consulting company with professionals from several industries. Some of the industries we cover include medical devices, pharmaceutical manufacturers, science and engineering, electronic components, industrial equipment, technology and communication, cars and automobiles, chemical products and substances, general merchandise, beverages, personal care, and automated systems. To mention a few, we provide market-verified industry estimations, technical trend analysis, crucial market research, strategic advice, competition analysis, production and demand analysis, and client impact studies.
Contact Maximize Market Research:
3rd Floor, Navale IT Park, Phase 2
Pune Banglore Highway, Narhe,
Pune, Maharashtra 411041, India
sales@maximizemarketresearch.com
+91 96071 95908, +91 9607365656
Media Contact
Company Name: MAXIMIZE MARKET RESEARCH PVT. LTD.
Contact Person: Geeta Yevle
Email: Send Email
Address:3rd Floor, Navale IT Park, Phase 2, Pune Banglore Highway, Narhe,
City: Pune
State: Maharashtra
Country: India
Website: https://www.maximizemarketresearch.com/market-report/in-vitro-diagnostics-quality-control-market/161874/